Abstract
Purpose. Insulin detemir has been found in clinical trials to be absorbed with very low variability. A series of experiments were performed to elucidate the underlying mechanisms.
Methods. The disappearance from an injected subcutaneous depot and elimination studies in plasma were carried out in pigs. Size-exclusion chromatography was used to assess the self-association and albumin binding states of insulin detemir and analogs.
Results. Disappearance T50% from the injection depot was 10.2 ± 1.2 h for insulin detemir and 2.0 ± 0.1 h for a monomeric acylated insulin analog. Self-association of acylated insulin analogs with same albumin affinity in saline correlated with disappearance rate and addition of albumin to saline showed a combination of insulin detemir self association and albumin binding. Intravenous kinetic studies showed that the clearance and volume of distribution decreased with increasing albumin binding affinity of different acylated insulin analogs.
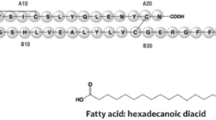
Conclusions. The protracted action of detemir is primarily achieved through slow absorption into blood. Dihexamerization and albumin binding of hexameric and dimeric detemir prolongs residence time at the injection depot. Some further retention of detemir occurs in the circulation where albumin binding causes buffering of insulin concentration. Insulin detemir provides a novel principle of protraction, enabling increased predictability of basal insulin.
Similar content being viewed by others
REFERENCES
S. O. Emdin, G. G. Dodson, J. M. Cutfield, and S. M. Cutfield. Role of zinc in insulin biosynthesis. Some possible zinc-insulin interactions in the pancreatic B-cell. Diabetologia 19:174–182 (1980).
S. Kang, J. Brange, A. Burch, A. Volund, and D. R. Owens. Subcutaneous insulin absorption explained by insulin's physicochemial properties. Evidence from absorption studies of soluble human insulin and insulin analogues in humans. Diabetes Care 14:942–948 (1991).
P. Hildebrandt, P. Sejrsen, S. L. Nielsen, and K. Birch. and L. Sestoft Diffusion and polymerisation determine the insulin absorption from subcutaneous tissue in diabetic patients. Scand. J. Clin. Lab. Invest. 45:685–690 (1985).
J. Brange, U. Ribel, J. F. Hansen, G. Dodson, M. T. Hansen, S. Havelund, S. G. Melberg, F. Norris, K. Norris, L. Snel, A. R. Sørensen, and H. O. Voigt. Monomeric insulins obtained by protein engineering and their medical implications. Nature 333:679–682 (1988).
T. Lauritzen, O. K. Faber, and C. Binder. Variation in insulin absorption and blood glucose concentrations. Diabetologia 17: 291–295 (1979).
P. Hildebrandt. Subcutaneous absorption of insulin in insulindependent diabetic patients. Dan. Med. Bull. 38:337–346 (1991).
J. Brange, D. R. Owens, S. Kang, and A. Volund. Monomeric insulins and their experimental and clinical implications. Diabetes Care 13:923–954 (1990).
J. Brange and A. Volund. Insulin analogs with improved pharmacokinetic profiles. Adv. Drug Deliv. Rev. 35:307–335 (1999).
G. B. Bolli. Physiological insulin replacement in type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 109:S317–S332 (2001).
A. M. Gualandi-Signorini and G. Giorgi. Insulin formulations-a review. Eur. Rev. Med. Pharmacol. Sci. 5(3):73–83 (2001).
P. Home. Insulin glargine: the first clinically useful extendedacting insulin in half a century? Exp. Opin. Invest. Drugs 8:307–314 (1999).
G. B. Bolli and D. R. Owens. Insulin glargine. Lancet 356:443–445 (2000).
D. R. Owens, B. Zinman, and G. B. Bolli. Insulins today and beyond. Lancet 358:739–746 (2001).
K. Hermansen, S. Madsbad, H. Perrild, A. Kristensen, and M. Axelsen. Comparison of the soluble basal insulin analog insulin detemir with NPH insulin. A randomised open crossover trial in type 1 diabetic subjects on basal-bolus therapy. Diabetes Care 24:296–301 (2001).
D. R. Owens, P. A. Coates, S. D. Luzio, J. P. Tinbergen, and R. Kurzhals. Pharmacokinetics of 125I-labelled insulin glargine (HOE 901) in healthy men. Comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care 23:813–819 (2000).
J. Markussen, P. Hougaard, U. Ribel, A. R. Sørensen, and E. Sørensen. Soluble, prolonged-acting insulin derivatives, I: Degree of protraction and crystallizability of insulins substituted in positions A17, B8, B23, B27 and B30. Protein Eng. 1:205–213 (1987).
J. Markussen, I. Diers, A. Engesgaard, M. T. Hansen, P. Hougaard, L. Langkjaer, K. Norris, U. Ribel, L. Snel, A. R. Sørensen, and H. O. Voigt. Soluble, prolonged-acting insulin derivatives. II. Degree of protraction and crystallizability of insulins substituted in positions A17, B8, B13, B27 and B30. Protein Eng. 1:215–223 (1987).
G. B. Bolli, R. D. Di Marchi, G. D. Park, S. Pramming, and V. A. Koivisto. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia 42(10):1151–1167 (1999).
L. Heinemann, R. Linkeschova, K. Rave, B. Hompesch, M. Sedlak, and T. Heise. Time-action profile of the long-acting insulin analogue insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 23:644–649 (2000).
M. Lepore, S. Pampanelli, C. Fanelli, F. Porcellati, L. Bartocci, A. Di Vincenzo, C. Cordoni, E. Costa, P. Brunetti, and G. B. Bolli. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 29:2142–2148 (2000).
P. Kurtzhals, S. Havelund, I. Jonassen, B. Kiehr, U. D. Larsen, U. Ribel, and J. Markussen. Albumin binding of insulin acylated with fatty acids: characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effects in vivo. Biochem. J. 312:725–731 (1995).
J. Markussen, S. Havelund, P. Kurtzhals, A. S. Andersen, J. Halstrom, E. Hasselager, U. D. Larsen, U. Ribel, L. Schäffer, K. Vad, and I. Jonassen. Soluble fatty acid insulins bind to albumin and show protracted action in pigs. Diabetologia 39:281–288 (1996).
P. Kurtzhals, S. Havelund, I. Jonassen, and J. Markussen. Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. J. Pharm. Sci. 86:1365–1368 (1997).
L. Heinemann, K. Sinha, C. Weyer, M. Loftager, S. Hirschberger, and T. Heise. Time-action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabet. Med. 16:332–338 (1999).
G. A. Brunner, G. Sendhofer, A. Wutte, M. Ellmerer, B. Sogaard, A. Siebenhofer, S. Hirschberger, G. J. Krejs, and T. R. Pieber. Pharmacokinetic and pharmacodynamic properties of long-acting insulin analogue NN304 in comparison to NPH insulin in humans. Exp. Clin. Endocrinol. Diabetes 108:100–105 (2000).
M. Hamilton-Wessler, M. Ader, M. Dea, D. Moore, P. N. Jorgensen, J. Markussen, and R. N. Bergman. Mechanism of protracted metabolic effects of fatty acid acylated insulin, NN304, in dogs: retention of NN304 by albumin. Diabetologia 42:1254–1263 (1999).
M. Hamilton-Wessler, M. Ader, M. K. Dea, D. Moore, M. Loftager, J. Markussen, and R. N. Bergman. Mode of transcapillary transport of insulin and insulin analog NN304 in dog hindlimb. Evidence for passive diffusion. Diabetes 51:574–582 (2002).
M. K. Dea, M. Hamilton-Wessler, M. Ader, D. Moore, L. Schaffer, M. Loftager, A. Volund, and R. N. Bergman. Albumin binding of acylated insulin (NN304) does not deter action to stimulate glucose uptake. Diabetes 51(3):762–769 (2002).
S. Havelund, J. B. Halstrøm, I. Jonassen, A. S. Andersen, and J. Markussen. Acylated insulin. European Patent EP 0 792 290 B1 (2002).
K. Drejer, V. Kruse, U. D. Larsen, P. Hougaard, S. Bjørn, and S. Gammeltoft. Receptor binding and tyrosine kinase activation by insulin analogues with extreme affinities studied in human hepatoma HepG2 cells. Diabetes 40:1488–1495 (1991).
P. Kurtzhals and U. Ribel. A novel principle of protraction of potential use for basal insulin delivery. Diabetes 44:1381–1385 (1995).
J. L. Whittingham, S. Havelund, and I Jonassen. Crystal structure of a prolonged-acting insulin with albumin-binding properties. Biochemistry 36:2826–2831 (1997).
H. B. Olsen and N. C. Kaarsholm. Structural effects of protein lipidation as revealed by LysB29-myristoyl, des(B30) insulin. Biochemistry 39:11893–11900 (2000).
H. B. Olsen, S. Ludvigsen, and N. C. Kaarsholm. The relationship between insulin bioactivity and structure in the NH2-terminal A-chain helix. J. Mol. Biol. 284:477–488 (1998).
M. Ellmerer, L. Schaupp, G. A. Brunner, G. Sendlhofer, A. Wuffe, P. Wach, and T. R. Pieber. Measurement of interstitial albumin in human skeletal muscle and adipose tissue by openflow microperfusion. Am. J. Physiol. Endocrinol. Metab. 278:E352–E356 (2000).
U. Ribel, K. Jørgensen, and U. Henriksen. The pig as a model for subcutaneous absorption in man. In: M. Serano-Rios and P. J. Lefebvre (eds.), Diabetes. Proceeding of 12th Congress of the International Diabetes Federation, Madrid 1985. Excerpta Medica, Amsterdam, 1986, pp. 891-896.
P. Vague, J.-L. Selam, S. Skeie, I. D. Leeuw, J. W. F. Elte, H. Haahr, A. Kristensen, and E. Draeger. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care 26:590–596 (2003).
L. Heinemann, K. Sinha, C. Weyer, M. Loftager, S. Hirschberger, and T. Heise. Time-action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabet. Med. 16:332–338 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Havelund, S., Plum, A., Ribel, U. et al. The Mechanism of Protraction of Insulin Detemir, a Long-Acting, Acylated Analog of Human Insulin. Pharm Res 21, 1498–1504 (2004). https://doi.org/10.1023/B:PHAM.0000036926.54824.37
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000036926.54824.37