Abstract
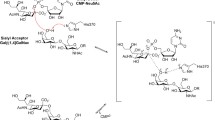
Inhibitors that are structurally related to the transition-state model of the proposed SN1-type mechanism of sialyl transfer, exhibit particularly high binding affinities to α(2-6)sialyltransferases. Furthermore, replacing the neuraminyl residue with a simple aryl or hetaryl ring and substituting the carboxylate group for a phosphonate moiety, improves both binding affinity and synthetic accessibility. Herein we report on the synthesis and inhibition of a wide range of novel, potent transition-state analogue based α(2-6)sialyltransferase inhibitors comprising a planar anomeric carbon, an increased distance between the anomeric carbon and the CMP leaving group, and at least two negative charges. We also present a short, efficient asymmetric synthesis of the most promising benzyl inhibitors, providing rapid access to large quantities of highly potent, stereochemically-pure (>96% de) inhibitors for further biological investigation (e.g. (R)-3b, K i = 70 nM). Published in 2003.
Similar content being viewed by others
References
Morgenthaler J, Kemmner J, Brossmer R, Sialic acid dependent cell adhesion to collagen IV correlates with in vivotumorigenicity of the human colon carcinoma sublines HCT116, HCT116a and HCT116b, Biochem Biophys Res Commun 171, 860-6 (1990).
Rosenberg A, Biology of Sialic Acids (Plenum New York, 1995).
Reutter W, Stäsche R, Stehling P, Baum O, in Glycosciences, edited by Gabius HJ, Gabius S (Chapman and Hall, Weinheim, 1997), pp. 245-9.
Mammen M, Choi S-K, Whitesides GM, Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors, Angew Chem 110, 2908-58 (1998); Angew Chem Int Ed 37, 2754-94 (1998).
von Itzstein M, Colman P, Design and synthesis of carbohydrate-based inhibitors of protein-carbohydrate interactions, Curr Opin Struct Biol 6, 703-9 (1996).
Schauer R, Achievements and challenges of sialic acid research, Glycoconjugate J. 17, 485-99 (2000).
Dall'Olio F, Chiricolo M, Sialyltransferases in cancer, Glycocon-jugate J. 18, 841-50 (2001).
Hennet T, Chui D, Paulson JC, Marth JD, Immune regulation by the ST6Gal sialyltransferase, Proc Natl Acad Sci 95, 4504-9 (1998).
Jung K-H, Schmidt RR, Glycosyltransferase inhibitors. In Carbohydrate-based Drug Discovery, edited by Wong CH, (Wiley-VCH, 2003), pp. 609-59.
Schaub C, Müller B, Schmidt RR, New sialyltransferase inhibitors based on CMP-quinic acid-development of a new sialyltrans-ferase assay, Glycoconjugate J. 15, 345-54 (1998); and references therein.
Müller B, Martin TJ, Schaub C, Schmidt RR, Synthesis of phos-phonate analogues of CMP-Neu5Ac-determination of α (2-6)-sialyltransferase inhibition, Tetrahedron Lett 39, 509-12 (1998).
Amann F, Schaub C, Müller B, Schmidt RR, New potent sialyl-transferase inhibitors-synthesis of donor and of transition-state analogues of sialyl donor CMP-Neu5Ac, Chem Eur J 4, 1106-15 (1998).
Müller B, Schaub C, Schmidt RR, Efficient sialyltransferase in-hibitors based on the generation of transition-state analogues of the sialyl donor, Angew Chem 110, 3021-4 (1998); Angew Chem Int Ed 37, 2893-7 (1998).
Schwörer R, Schmidt RR, Efficient sialyltransferase inhibitors based on glycosides of N-acetylglucosamine, J AmChem Soc 124, 1632-7 (2002).
Skropeta D, Schwörer R, Schmidt RR, Stereoselective synthesis of phosphoramidate α (2-6)sialyltransferase transition-state analogue inhibitors, Bioorg Med Chem Lett 3351-4 (2003).
Bruner M, Horenstein BA, Use of an altered sugar-nucleotide to unmask the transition state for α (2→6) sialyltransferase, Biochemistry 39, 2261-8 (2000).
Horenstein BA, Bruner M, Isotope trapping and kinetic isotope effect studies of rat liver α (2→6) sialyltransferase, Biochemistry 37, 289-97 (1998).
Horenstein BA, Bruner M, Acid-catalyzed solvolysis of CMP-N-acetyl Neuraminate: Evidence for a sialyl cation with a finite lifetime, J AmChem Soc 118, 10371-9 (1996).
Schröder PN, Giannis A, From substrate to transition state ana-logues: The first potent inhibitor of sialyltransferases, AngewChem 111, 1471-2 (1999); Angew Chem Int Ed 38, 1379-80 (1999).
Schaub C, Müller B, Schmidt RR, Sialyltransferase inhibitors based on CMP-quinic acid, Eur J Org Chem 1745-58 (2001).
Skropeta D, Schmidt RR, Chiral, Non-racemic α-hydroxyphosphonates and phosphonic acids via stereoselective hydroxylation of diallyl benzylphosphonates, Tetrahedron: Asymmetry 265-73 (2003).
Wiemer DF, Synthesis of nonracemic phosphonates, Tetrahedron 53, 16609-44 (1997).
Groeger H, Hammer B, Catalytic concepts for the enantioselective synthesis of α-amino and α-hydroxy phosphonates, Chem Eur J 6, 943-8 (2000).
Rowe BJ, Spilling CD, The synthesis of 1-hydroxy phosphonates of high enantiomeric excess using sequential asymmetric reac-tions: Titanium alkoxide-catalyzed P-C bond formation and kinetic resolution, Tetrahedron: Asymmetry 12, 1701-8 (2001).
Yokomatsu T, Yamagishi T, Shibuya S, Stereodivergent synthesis of â-amino-α-hydroxyphosphonic acid derivatives by lewis acid mediated stereoselective hydrophosphonylation of α-amino alde-hydes, Tetrahedron: Asymmetry 4, 1401-4 (1993).
Cermak DM, Yanming D, Wiemer DF, Synthesis of non-racemic dimethyl α-(hydroxyfarnesyl)phosphonates via oxi-dation of dimethyl farnesylphosphonate with (camphorsul-fonyl) oxaziridines, J Org Chem 64, 388-93 (1999).
Hammerschmidt F, Lindner W, Wuggenig F, Zarbl E, En-zymes in organic chemistry. Chemo-enzymatic synthesis of L-phosphaserine and L-phosphaisoserine and enantioseparation of amino-hydroxyethylphosphonic acids by non-aqueous capillary electrophoresis with quinine carbamate as chiral ion pair agent, Tetrahedron: Asymmetry 11, 2955-64 (2000).
Pogatchnik DM, Wiemer DF, Enantioselective synthesis of α-hydroxy phosphonates via oxidation with (camphorsul-fonyl) oxaziridines, Tetrahedron Lett 38, 3495-8 (1997).
Kunz H, Unverzagt C, The allyloxycarbonyl (Aloc) Moiety-conversion of an unsuitable into a valuable protecting group for peptide synthesis, Angew Chem 96, 426-7 (1984); Angew Chem, Int Ed Engl 23,36(1984).
Chappell MD, Halcomb RL, Synthesis of CMP-sialic acid conjugates: Substrates for the enzymatic synthesis of natural and designed sialyl oligosaccharides, Tetrahedron 53, 11109-20 (1997).
Vanderhaeghe H, Claesen M, Pyrimidines III-Dérivés de l'acide pirimidone-6-carboxylique-4 et de l'aldéhyde correspondant, Bull Soc Chim Belg 66, 292-302 (1957).
Cretcher TB, Reserrches on pyrimidines. LXXV. pyrimidine alde-hydes and their biochemical interest (Thiouracilaldehyde), J Am Chem Soc 37, 2144-51 (1915).
Still WC, Kahn M, Mitra A, Rapid chromatographic technique for preparative separations with moderate resolution, J Org Chem 43, 2923-5 (1978).
Rights and permissions
About this article
Cite this article
Skropeta, D., Schwörer, R., Haag, T. et al. Asymmetric synthesis and affinity of potent sialyltransferase inhibitors based on transition-state analogues. Glycoconj J 21, 205–219 (2004). https://doi.org/10.1023/B:GLYC.0000045093.96413.62
Issue Date:
DOI: https://doi.org/10.1023/B:GLYC.0000045093.96413.62