Abstract
Purpose. To monitor the phase transitions during freeze-drying of disodium hydrogen phosphate.
Methods. The variable temperature sample stage of the X-ray diffractometer (XRD) was attached to a vacuum pump, which enabled the entire freeze-drying process to be carried out in the sample chamber. The phase transitions during the freeze-drying cycle were monitored in real time by XRD. Aqueous buffer solution (containing disodium hydrogen phosphate and sodium dihydrogen phosphate) was cooled at 2°C/min from room temperature to −70°C. It was then heated to −25°C and subjected to primary drying for 2 h at a chamber pressure of ∼100 mTorr, followed by secondary drying at −10°C.
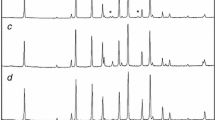
Results. In the frozen solution, disodium hydrogen phosphate had crystallized as the dodecahydrate (Na2HPO4⋅12H2O) as was evident from its characteristic lines at ∼5.37, 4.27, and 2.81 Å. Primary drying for 2 h resulted in ice sublimation, and the complete disappearance of the dodecahydrate peaks.
Conclusion. The dehydration of the crystalline dodecahydrate resulted in an amorphous anhydrate. Thus the amorphous nature of the end product is a result of phase transitions during the process and do not reflect the solid-state of the ingredients during the entire process.
Similar content being viewed by others
REFERENCES
J. F. Carpenter, M. J. Pikal, B. S. Chang, and T. W. Randolph. Rational design of stable lyophilized protein formulations: some practical advice. Pharm. Res. 14:969-975 (1997).
L. Van den Berg and D. Rose. Effect of freezing on the pH and composition of sodium and potassium phosphate solutions; the reciprocal system KH2PO4-Na2HPO4-H2O. Arch. Biochem. Biophys. 81:319-329 (1959).
N. Murase and F. Franks. Salt precipitation during the freeze-concentration of phosphate buffer solutions Biophys. Chem. 34:293-300 (1989).
G. Gomez, M. J. Pikal, and N. Rodriguez-Hornedo. Effect of initial buffer composition on pH changes during far-from-equilibrium freezing of sodium phosphate buffer solutions. Pharm. Res. 18:90-97 (2001).
R. K. Cavatur and R. Suryanarayanan. Characterization of frozen aqueous solutions by low temperature X-ray powder diffractometry. Pharm. Res. 15:194-199 (1998).
W. E. Garner. The kinetics of endothermic solid reactions. In W. E. Garner (ed.), Chemistry of the Solid State, Academic Press, New York 1955 pp. 213-231.
Y. Li, J. Han, G. G. Z. Zhang, D. J. W. Grant, and R. Suryanarayanan. In situ dehydration of carbamazepine dihydrate: a novel technique to prepare amorphous anhydrous carbamazepine. Pharm. Dev. Technol. 5:257-266 (2000).
A. Saleki-Gerhardt, J. G. Stowell, S. R. Byrn, and G. Zografi. Hydration and dehydration of crystalline and amorphous forms of raffinose. J. Pharm. Sci. 84:318-323 (1995).
K. Chatterjee, E. Y. Shalaev, and R. Suryanarayanan. Phase transitions in raffinose solutions in frozen state and during freeze-drying: practical implications. Poster presented at the Annual AAPS meeting, Toronto, 2002.
A. Pyne and R. Suryanarayanan. The effect of additives on the crystallization of cefazolin sodium during freeze-drying. Pharm. Res. 20:280-288 (2003).
G. Gomez. Crystallization-related pH changes during freezing of sodium phosphate buffer solutions. PhD Thesis, University of Michigan, 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pyne, A., Chatterjee, K. & Suryanarayanan, R. Crystalline to Amorphous Transition of Disodium Hydrogen Phosphate During Primary Drying. Pharm Res 20, 802–803 (2003). https://doi.org/10.1023/A:1023445905372
Issue Date:
DOI: https://doi.org/10.1023/A:1023445905372