Abstract
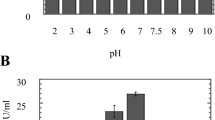
Chrysosporium keratinophilum IMI 338142 isolated from a waste site containing organopollutants was studied for its ability to produce extracellular proteases on glucose-gelatin medium. Fungus was observed to be a potent producer of such enzymes. Enzyme secretion was best at 15 days of incubation period at pH 8 and temperature 40 °C. Asparagine was repressive to protease expression. No relationship existed between the enzyme yield and increase in biomass. Exogenous sugars suppressed enzyme production in the descending order as follows: glucose > arabinose > maltose > mannose > fructose. The enzyme released showed the ability to decompose two keratin substrates tested. Buffalo skin was the most actively degraded substrate when exogenous glucose was absent. Presence of glucose suppressed both enzyme production and degradation of keratin. However, the rate of keratin degradation was independent of enzyme production.
Similar content being viewed by others
References
Anglesea D, Swift MJ. The keratinophilic ability of Monodictys levis and Trichophyton ajelloi. Trans Br Mycol Soc 1971; 57: 333–337.
Kunert J. Keratin decomposition by dermatophytes. I. Sulphite production as a possible way of substrate denaturation. Z Allg Mikrobiol 1973; 15: 59–71.
Kunert J. Biochemical mechanism of keratin degradation by the actinomycete Streptomyces fradiae and the fungus Microsporum gypseum: A comparison. J Basic Microbiol 1989; 29: 597–604.
Meevootisom V, Niederpruem DJ. Control of extracellular proteases in dermatophytes and especially Trichophyton rubrum. Sabouraudia 1979; 17: 91–106.
Chesters CGC, Mathison GE. The decomposition of wool keratin by Keratinomyces ajelloi. Sabouraudia 1963; 2: 225–237.
Deshmukh SK, Agrawal SC. In vitro degradation of human hair by some keratinophilic fungi. Mykosen 1982; 25: 454–458.
Deshmukh SK, Agrawal SC. Degradation of human hair by some dermatophytes and other keratinophilic fungi. Mykosen 1985; 28: 463–466.
Evans EGV, Hose H. In vitro degradation of human hair by Hendersonula toruloidea. Sabouraudia 1975; 13: 323–328.
Kushwaha RKS. The in vitro degradation of peacock feathers by some fungi. Mykosen 1983; 26: 324–326.
Nigam N, Kushwaha RKS. Decomposition of feathers and hairs by keratinophilic fungi. Indian J Microbiol 1989; 29: 241–244.
Safranek WW, Goos RD. Degradation of wool by saprotrophic fungi. Canadian J Microbiol 1982; 28: 137–140.
Kornillowicz-Kowalska T. Studies on the decomposition of keratin wastes by saprotrophic microfungi. I. Criteria for evaluating keratinolytic activity. Acta Mycologica 1997; 32: 51–79.
Kornillowicz-Kowalska T. Studies on the decomposition of keratin wastes by saprotrophic microfungi. II. Sulphur and nitrogen balance. Acta Mycologica 1997; 32: 81–93.
Singh CJ, Singh BG. Characterization of extracellular proteolytic enzyme of Chrysosporium tropicum CF34 and its role in keratin degradation. Ind J Microbiol 1995; 35: 311–315.
Singh CJ, Singh BG, Singh BS. Biodegradation of certain keratin substrates in vitro by some keratinophilic fungi. Ad Plant Sci 1995; 8: 271–276.
Singh CJ. Characterization of an extracellular keratinase of Trichophyton simii and its role in keratin degradation. Mycopathologia 1997; 137: 13–16.
Singh CJ. Exocellular proteases of Malbranchea gypsea and their role in keratin deterioration. Mycopathologia 1999; 143: 147–150.
Vanbreusegham R. Technique biologique pour l'isolement des dermatophytes du sol. Ann Soc Belg Med Trop 1952; 32: 173–178.
Meyers SP, Ahearn DG. Extracellular proteolysis of Candida lipolytica. Mycologia 1977; 69: 646–651.
Fergus CL. The production of amylase by some thermophilic fungi. Mycologia 1969; 61: 1171–1175.
Ziegler H, Bohme H, Reichmann G. Stoffwechselphysiologische Untersuchungen uber den Abbau von Proteinen durch Microsporum gypseum und M. canis. Dermatol Mschr 1969; 155: 835–856.
Leighton TJ, Stock JJ. Biochemical changes during fungal sporulation and spore germination. I. Phenyl methyl sulphonyl fluoride inhibition of macroconidial germination in Microsporum gypseum. J Bacteriol 1970; 101: 931–940.
Ruffin P, Van Brussel E, Biguet J, Biserte G. Caracterisation partielle de deux aminopeptidases extracellulaires du dermatophyte Keratinomyces ajelloi. Biochemie 1979; 16: 495–500.
Takiuchi I, Higuchi D, Sei Y, Koga M. Isolation of an extracellular proteinase (keratinase) from Microsporum canis. Sabouraudia 1982; 20: 281–288.
Sanyal AK, Das SK, Banerjee AB. Purification and partial characterization of an exocellular proteinase from Trichophyton rubrum. Sabouraudia 1985; 23: 165–178.
El-Naghy MA, El-Ktatny MS, Fadl-Allah EM, Nazeer WW. Degradation of chicken feathers by Chrysosporium georgiae. Mycopathologia 1999; 143: 77–84.
Srivastava JN, Ghawana VK, Kumar A. Biodegradation of wool by Trichophyton simii and Aspergillus niger. Mycoses 1996; 39: 483–487.
Day WC, Toncic P, Stratman SL, Leeman U, Harmon SR. Isolation and properties of an extracellular protease of Trichophyton granulosum. Biochemica et Biophysica Acta 1968; 167: 597–606.
Abdel-Rahman SM. Polymorphic exocellular protease expression in clinical isolates of Trichophyton tonsurans. Mycopathologia 2001; 150: 117–120.
Muhsin TM, Salih TH. Exocellular enzyme activity of dermatophytes and other fungi isolated from ruminants and southern Iraq. Mycopathologia 2001; 150: 49–52.
Muhsin TM, Aubaid AH. Partial purification and some biochemical characteristics of exocellular keratinase from Trichophyton mentagrophytes var. erinacei. Mycopathologia 2001; 150: 121–125.
Brouta F, Descamps F, Fett T, Losson B, Gerday C, Mignon B. Purification and characterization of a 43.5 kDa keratinolytic metalloprotease from Microsporum canis. Med Mycol 2001; 39: 269–275.
Viani FC, Santos JID, Paula CR, Larson CE, Gambale W. Production of extracellular enzymes by Microsporum canis and their role in its virulence. Med Mycol 2001; 39: 463–468.
McCarrol DR, Thore E. Pectolytic, cellulolytic and proteolytic activities expressed by cultures of Endothia parasitica and inhibition of these activities by components extracted from Chinese and American chestnut inner bark. Physiol Plant Pathol 1985; 26: 326–378.
Oyeka CA, Gugnani HC. Extracellular proteases of Hendersonula toruloidea, Scytalidium hyalinum and Scytalidium japonicum. Mycopathologia 1995; 130: 67–70.
O'Sullivan J, Mathison GE. The localization and secretion of proteolytic enzyme complex by the dermatophytic fungus Microsporum canis. J Gen Microbiol 1971; 68: 319–326.
Hansen MA, Marzluf GA. Regulation of a sulphur controlled protease in Neurospora crassa. J Bacteriol 1973, 116: 785–789.
Rippon JW. Elastase: Production by ringworm fungi. Science 1967; 157: 947.
Rippon JW, Varadi DP. The elastase of pathogenic fungi and actinomycetes. J Invest Dermatol 1968; 50: 54–58.
Hopsu-Havu VK, Sonck CE, Tunnela E. Production of elastase by pathogenic and non-pathogenic fungi. Mykosen 1972, 15: 105–110.
Abdelal ATH, Kennedy EH, Ahearn DG. Purification and characterization of a neutral protease from Saccharomycopsis lipolytica. J Bacteriol 1977; 130: 1125–1129.
Drucker H. Regulation of exocellular proteases in Neurospora crassa: Induction and repression of enzyme synthesis. J Bacteriol 1972; 110: 1041–1049.
Klapper BF, Jameson DM, Mayer RM. Factors affecting the synthesis and release of the extracellular protease of Aspergillus oryzae NRRL 2160. Biochemica et Biophysica Acta 1973; 304: 513–519.
Wang MC, Bartinicki-Garcia S. Synthesis of β-1,3-glucan microfibrils by a cell free extract from Phytophthora cinnamomi. Arch Biochem Biophys 1976; 175: 351–354.
Somkuti GA, Babel FJ. Conditions influencing the synthesis of acid protease by Mucor pusillus Lindt. App Microbiol 1967; 15: 1309–1312.
Van Wicz R, Konijn TM. Cyclic 3',5'-AMP in Sachharomyces carlsbergensis under various conditions of catabolite repression. FEBS Letters 1971; 13: 184–186.
Ulfig K, Lukasik W. Guarro I, Cano I, Gene I, Vidal P, Figueras MJ. The seasonal changes of keratinolytic fungi in sediments of Catalonian rivers (Spain). Water Air Soil Poll 1997; 96: 269–290.
Ulfig K, Guarro J, Cano J, Gene J, Vidal P. Figueras MJ, Lukasik W. The occurrence of keratinolytic fungi in sediments of the river Tordera (Spain). FEMS Microbiol Ecol 1997; 22: 111–117.
Ramesh VM, Hilda A. Incidence of keratinophilic fungi in the soils of primary schools and public parks of Madras city, India. Mycopathologia 1999; 143: 139–145.
Kaul S, Sumbali G. Impact of some ecological factors on the occurrence of poultry soil-inhabiting keratinophiles. Mycopathologia 1999; 143: 155–159.
Kaul S, Sumbali G. Keratinolysis by poultry farm soil fungi. Mycopathologia 1997; 139: 137–140.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, C.J. Optimization of an extracellular protease of Chrysosporium keratinophilum and its potential in bioremediation of keratinic wastes. Mycopathologia 156, 151–156 (2003). https://doi.org/10.1023/A:1023395409746
Issue Date:
DOI: https://doi.org/10.1023/A:1023395409746