Abstract
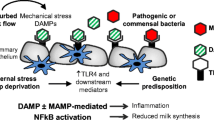
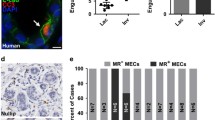
The processes by which the involuting mammary gland clears residual milk and milk fat, as well as apoptotic cells, have gone largely unstudied in the modern literature. Here we review the evidence for and against the involvement of professional phagocytes of hematopoetic lineage in this process. Additionally we present evidence that mammary epithelial cells themselves are capable of phagocytosis and may be responsible for the majority of apoptotic cell and residual milk clearance during murine involution. In this scheme these cells regulate their cytokine production in response to apoptotic cells in a manner similar to other cells, including macrophages. The ensuing model describes a process of involution that actively suppresses an inflammatory response in the gland, allowing for effective tissue remodeling and damage prevention.
Similar content being viewed by others
REFERENCES
L. M. A. Maeder (1922). Changes in the mammary gland of the albino rat (mus norvegicus albinus) during lactation and involution. Am.J.Anat. 31: 1–26.
W. L. Williams (1942). Normal and experimental mammary involution in the mouse as related to the inception and cessa-tion of lactation. Am.J.Anat. 71: 1–41.
S. R. Wellings and K. B. DeOme (1963). Electron microscopy of milk secretion in the mammary gland of the c3h/crgl mouse. III. Cytomorphology of the involuting gland. J.Natl.Cancer Inst. 30: 241–267.
K. K. Sekhri, D. R. Pitelka, and K. B. DeOme (1967). Studies of mouse mammary glands. I. Cytomorphology of the normal mammary gland. J.Natl.Cancer Inst. 39: 459–490.
R. C. Richards and G. K. Benson (1971). Ultrastructural changes accompanying involution of the mammary gland in the albino rat. J.Endocrinol. 51: 127–135.
C. Alexander, S. Selvarajan, J. Mudgett, and Z. Werb (2001). Stromelysin-1 regulates adipogenesis during mammary gland involution. J.Cell Biol. 152: 693–703.
V. Gouon-Evans, M. E. Rothenberg, and J. W. Pollard (2000). Postnatal mammary gland development requires macrophages and eosinophils. Development 127: 2269–2282.
C. Wilde, C. Knight, and D. Flint (1999). Control of milk se-cretion and apoptosis during mammary involution. J.Mam. Gland Biol.Neoplasia 4: 129–136.
M. Paape, K. Shafer-Weaver, A. Capuco, K. Oostveldt, and C. Burvenich (2000). Immune surveillance of mammary tissue by phagocytic cells. Adv.Exp.Med.Biol. 480: 259–277.
C. S. Lee, G. H. McDowell, and A. K. Lascelles (1969). The importance of macrophages in the removal of fat from the involuting mammary gland. Res.Vet.Sci. 10: 34–38.
W. Nordin and C. S. Lee (1982). Cytology of milk in guinea pigs. Acta Anat. 113: 135–144.
C. S. Lee, I. McCauley, and P. E. Hartmann (1983). Light and electron microscopy of cells in pig colostrum, milk and invo-lution secretion. Acta Anat. 116: 126–135.
I. G. Colditz (1988). Studies on the inflammatory response during involution of the ovine mammary gland. Q.J. Exp. Physiol. 73: 363–368.
L. Tatarczuch, C. Philip, and C. S. Lee (2000). Leucocyte phe-notypes in involuting and fully involuted mammary glandular tissues and secretions of sheep. J.Anat. 196: 313–326.
S. Nickerson (1989). Immunological aspects of mammary in-volution. J.Dairy Sci. 72: 1665–1678.
N. Manlongat, T. Yang, L. Hinckley, R. Bendel, and H. Krider (1998). Physiologic-chemoattractant-induced migration of polymorphonuclear leukocytes in milk. Clin.Diagn. Lab.Immunol. 5: 375–381.
M. Limon (1902). Phenomenes histologiques de la secre-tion lactee. Journal de l'anatomie et de la physiologie 38: 14–34.
S. Bratianu and C. Guerriero (1930). Sur le pouvoir phago-cytaire des cellules epitheliales de la glande mammaire. C.R. Hebd.Seanc.Acad.Sci.Paris 190: 1529–1530.
N. I. Walker, R. E. Bennet, and J. F. R. Kerr (1989). Cell death by apoptosis during involution of the lactating breast in mice and rats. Am.J.Anat. 185: 19–32.
H. J. Helminen and J. L. Ericsson (1968). Studies on mammary gland involution: II. Ultrastructural evidence for auto-and heterophagocytosis. J.Ultrastruct.Res. 25: 214–227.
H. J. Helminen and J. L. Ericsson (1971). Effects of enforced milk stasis on mammary gland epithelium, with special ref-erence to changes in lysosomes and lysosomal enzymes. Exp. Cell Res. 68: 411–427.
H. E. Mayberry (1964). Macrophages in post-secretory mam-mary involution in mice. Anat.Rec. 149: 99–112.
R. C. Richards and G. K. Benson (1971). Involvement of the macrophage system in the involution of the mammary gland in the albino rat. J.Endocrinol. 51: 149–156.
L. Tatarczuch, C. Philip, and C. S. Lee (1997). Involution of the sheep mammary gland. J.Anat. 190: 405–416.
J. Savill, V. Fadok, P. Henson, and C. Haslett (1993). Phagocyte recognition of cells undergoing apoptosis. Immunol.Today 14: 131–136.
H. J. Helminen and J. L. Ericsson (1968). Studies on mam-mary gland involution: I. On the ultrastructure of the lactating mammary gland. J.Ultrastruct.Res. 25: 193–213.
G. Chepko and G. H. Smith (1997). Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29: 239–253.
G. Chepko and G. H. Smith (1999). Mammary epithelial stem cells: Our current understanding. J.Mammary Gland Biol. Neoplasia 4: 35–52.
G. H. Smith and G. Chepko (2001). Mammary epithelial stem cells. Microsc.Res.Tech. 52: 190–203.
P. Masso-Welch, K. Darcy, N. Stangle-Castor, and M. Ip (2000). A developmental atlas of rat mammmary gland histology. J.Mammary Gland Biol.Neoplasia 5: 165–185.
R. Talhouk, M. Bissell, and Z. Werb (1992). Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J.Cell Biol. 118: 1271–1282.
L. R. Lund (1996). Two distinct phases of apoptosis in mammary gland involution: Proteinase-independent and dependent pathways. Development 122: 181–193.
M. Li, X. Liu, G. Robinson, U. Bar-Peled, K. U. Wagner, W. S. Young, L. Hennighausen, and P. A. Furth (1997). Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc.Natl. Acad.Sci.U.S.A. 94: 3425–3430.
R. Kumar, R. K. Vadlamudi, and L. Adam(2000). Apoptosis in mammary gland and cancer. Endocrine-Related Cancer 7: 257–269.
R. Strange, T. Metcalfe, L. Thackray, and M. Dang (2001). Apoptosis in normal and neoplastic mammary gland devel-opment. Microsc.Res.Tech. 52: 171–181.
J. Rosenblatt, M. C. Raff, and L. P. Cramer (2001). An epithe-lial cell destined for apoptosis signals its neighbors to extrude it by an actin-and myosin-dependent mechanism. Curr.Biol. 11: 1847–1857.
K. D. Walton, K. U. Wagner, E. B. Rucker, III, J. M. Shillingford, K. Miyoshi, and L. Hennighausen (2001). Con-ditional deletion of the bcl-x gene from mouse mammary ep-ithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech. Dev. 109: 281–293.
A. Marti, P. Ritter, R. Jager, H. Lazar, A. Baltzer, J. Schenkel, W. Declercq, P. Vandenabeele, and R. Jaggi (2001). Mouse mammary gland involution is associated with cytochrome c release and caspase activation. Mech.Dev. 104: 89–98.
L. Quarrie, C. Addey, and C. Wilde (1996). Programmed cell death during mammary tissue involution induced by weaning, litter removal, and milk stasis. J.Cell Physiol. 168: 559–569.
J. U. Schweichel and H. J. Merker (1973). The morphology of various types of cell death in prenatal tissues. Teratology 7: 253–266.
L. M. Schwartz, S. W. Smith, M. E. Jones, and B. A. Osborne (1993). Do all programmed cell deaths occur via apoptosis? Proc.Natl.Acad.Sci.U.S.A. 90: 980–984.
D. J. Klionsky and S. D. Emr (2000). Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721.
W. Bursch (2001). The autophagosomal-lysosomal compart-ment in programmed cell death. Cell Death Differ. 8: 569–581.
H. J. Helminen and J. L. Ericsson (1968). Studies on mammary gland involution: III. Alterations outside auto-and heterophagocytic pathways for cytoplasmic degradation. J.Ultrastruct.Res. 25: 228–239.
D. Brandes, E. Anton, and S. Barnard (1969). Lysosomes and cellular regressive changes in rat mammary gland involution. Lab.Invest. 20: 465–471.
E. I. Carlsson, B. W. Karlsson, and K. H. C. Waldemarson (1973). Dehydrogenases and nucleic acids in rat mammary gland during involution initiated at various stages of lactation. Comp.Biochem.Physiol.B 44: 93–108.
X. H. Liang, S. Jackson, M. Seaman, K. Brown, B. Kempkes, H. Hibshnoosh, and B. Levine (1999). Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402: 672–676.
S. Paglin, T. Hollister, T. Delohery, N. Hackett, M. McMahill, E. Sphicas, D. Domingo, and J. Yahalom (2001). A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 61: 439–444.
H. J. Helminen, J. L. Ericsson, and S. Orrenius (1968). Studies on mammary gland involution: IV. Histochemical and bio-chemical observations on alterations in lysosomes and lysoso-mal enzymes. J.Ultrastruct.Res. 25: 240–252.
K. U. Wagner, C. Boulanger, M. Henry, M. Sgagias, L. Hennighausen, and G. H. Smith (2002). An adjunct mammary epithelial cell population in parous females: Its role in func-tional adaptation and tissue renewal. Development 129: 1377–1386.
M. Berg, A. Dharmarajan, and B. Waddell (2002). Glucocor-ticoids and progesterone prevent apoptosis in the lactating rat mammary gland. Endocrinology 143: 222–227.
C. D. Gregory (2000). CD14-dependent clearance of apoptotic cells: Relevance to the immune system. Curr.Opin.Immunol. 12: 27–34.
V. A. Fadok, D. L. Bratton, and P. M. Henson (2001). Phago-cyte receptors for apoptotic cells: Recognition, uptake, and consequences. J.Clin.Invest. 108: 957–962.
V. A. Fadok and G. Chimini (2001). The phagocytosis of apop-totic cells. Semin.Immunol. 13: 365–372.
R. A. Schlegel and P. Williamson (2001). Phosphatidylserine, a death knell. Cell Death Differ. 8: 551–563.
F. Geske, J. Monks, L. Lehman, and V. Fadok (2002). The role of the macrophage in apoptosis: Hunter, gatherer and regulator. J.Hematol. 76: 16–26.
J. Savill, N. Hogg, and C. Haslett (1991). Macrophage vit-ronectin receptor, CD36, and thrombospondin cooperate in recognition of neutrophils undergoing programmed cell death. Chest 99: 6S–7S.
F. Takizawa, T. Shoutaro, and S. Nagasawa (1996). Enhance-ment of macrophage phagocytosis upon IC3b deposition on apoptotic cells. FEBS Lett. 397: 269–272.
K. Balasubramanian, J. Chandra, and A. J. Schroit (1997). Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition. J.Biol.Chem. 272: 31113–31117.
L. C. Korb and J. M. Ahearn (1997). C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: Complement deficiency and systemic lupus erythematosus re-visited. J.Immunol. 158: 4525–4528.
M. Botto, C. Dell'Agnola, A. E. Bygrave, E. M. Thompson, H. T. Cook, F. Petry, M. Loos, P. P. Pandolfi, and M. J. Walport (1998). Homozygous C1q deficiency causes glomerulonephri-tis associated with multiple apoptotic bodies [see comments]. Nat.Genet. 19: 56–59.
D. Mevorach, J. O. Mascarenhas, D. Gershov, and K. B. Elkon (1998). Complement-dependent clearance of apoptotic cells by human macrophages. J.Exp.Med. 188: 2313–2320.
D. Gershov, S. Kim, N. Brot, and K. B. Elkon (2000). C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: Im-plications for systemic autoimmunity. J.Exp.Med. 192: 1353–1364.
P. Rovere, G. Peri, F. Fazzini, B. Bottazzi, A. Doni, A. Bondanza, V. S. Zimmermann, C. Garlanda, U. Fascio, M. G. Sabbadini, C. Rugarli, A. Mantovani, and A. A. Manfredi (2000). The long pentraxin ptx3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 96: 4300–4306.
P. R. Taylor, A. Carugati, V. A. Fadok, H. T. Cook, M. Andrews, M. C. Carroll, J. S. Savill, P. M. Henson, M. Botto, and M. J. Walport (2000). A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp.Med. 192: 359–366.
C. A. Ogden, A. deCathelineau, P. R. Hoffmann, D. Bratton, B. Ghebrehiwet, V. A. Fadok, and P. M. Henson (2001). C1q and mannose binding lectin engagement of cell surface cal-reticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J.Exp.Med. 194: 781–795.
R. Hanayama, M. Tanaka, K. Miwa, A. Shinohara, A. Iwamatsu, and S. Nagata (2002). Identification of a factor that links apoptotic cells to phagocytes. Nature. 417: 182–187.
A. Moynault, M. F. Luciani, and G. Chimini (1998). ABC1, the mammalian homologue of the engulfment gene ced-7, is required during phagocytosis of both necrotic and apoptotic cells. Biochem.Soc.Trans. 26: 629–635.
Y. Hamon, C. Broccardo, O. Chambenoit, M. F. Luciani, F. Toti, S. Chaslin, J. M. Freyssinet, P. F. Devaux, J. McNeish, D. Marguet, and G. Chimini (2000). ABC1 promotes engulf-ment of apoptotic cells and transbilayer redistribution of phos-phatidylserine. Nat.Cell.Biol. 2: 399–406.
Y. Ishimoto, K. Ohashi, K. Mizuno, and T. Nakano (2000). Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J.Biochem. (Tokyo) 127: 411–417.
E. Nandrot, E. M. Dufour, A. C. Provost, M. O. Pequignot, S. Bonnel, K. Gogat, D. Marchant, C. Rouillac, B. Sepulchre de Conde, M. T. Bihoreau, C. Shaver, J. L. Dufier, C. Marsac, M. Lathrop, M. Menasche, and M. M. Abitbol (2000). Ho-mozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol.Dis. 7: 586–599.
M. O. Hall, A. L. Prieto, M. S. Obin, T. A. Abrams, B. L. Burgess, M. J. Heeb, and B. J. Agnew (2001). Outer segment phagocytosis by cultured retinal pigment epithelial cells re-quires gas6. Exp.Eye Res. 73: 509–520.
R. S. Scott, E. J. McMahon, S. M. Pop, E. A. Reap, R. Caricchio, P. L. Cohen, H. S. Earp, and G. K. Matsushima (2001). Phagocytosis and clearance of apoptotic cells is medi-ated by mer. Nature 411: 207–211.
E. Duvall, A. H. Wyllie, and R. G. Morris (1985). Macrophage recognition of cells undergoing programmed cell death (apop-tosis). Immunology 56: 351–358.
L. Dini, F. Autuori, A. Lentini, S. Oliverio, and M. Piacentini (1992). The clearance of apoptotic cells in the liver is mediated by the asialoglycoprotein receptor. FEBS Lett. 296: 174–178.
L. Falasca, A. Bergamini, A. Serafino, C. Balabaud, and L. Dini (1996). Human Kupffer cell recognition and phagocyto-sis of apoptotic peripheral blood lymphocytes. Exp.Cell Res. 224: 152–162.
L. Dini and E. C. Carla (1998). Hepatic sinusoidal endothe-lium heterogeneity with respect to the recognition of apoptotic cells. Exp.Cell Res. 240: 388–393.
M. Ruzittu, E. C. Carla, M. R. Montinari, G. Maietta, and L. Dini (1999). Modulation of cell surface expression of liver carbohydrate receptors during in vivo induction of apoptosis with lead nitrate. Cell Tissue Res. 298: 105–112.
C. D. Gregory, A. Devitt, and O. Moffatt (1998). Roles of ICAM-3 and CD14 in the recognition and phagocytosis of apoptotic cells by macrophages. Biochem.Soc.Trans. 26: 644–649.
O. D. Moffatt, A. Devitt, E. D. Bell, D. L. Simmons, and C. D. Gregory (1999). Macrophage recognition of ICAM-3 on apoptotic leukocytes. J.Immunol. 162: 6800–6810.
M. K. Callahan, P. Williamson, and R. A. Schlegel (2000). Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ 7: 645–653.
P. R. Hoffmann, A. M. deCathelineau, C. A. Ogden, Y. Leverrier, D. L. Bratton, D. L. Daleke, A. J. Ridley, V. A. Fadok, and P. M. Henson (2001). Phosphatidylserine (PS) in-duces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J.Cell Biol. 155: 649–660.
K. Kurosaka, N. Watanabe, and Y. Kobayashi (2001). Pro-duction of proinflammatory cytokines by resident tissue macrophages after phagocytosis of apoptotic cells. Cell Immunol. 211: 1–7.
V. A. Fadok, D. L. Bratton, D. M. Rose, A. Pearson, R. A. Ezekewitz, and P. M. Henson (2000). A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405: 85–90.
S. E. Hall, J. Savill, J. E. Henson, and C. Haslett (1994). Apop-totic neutrophils are phagocytosed by fibroblasts with partici-pation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J.Immunol. 153: 3218–3227.
M. R. Bennett, D. F. Gibson, S. M. Schwartz, and J. F. Tait (1995). Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phos-phatidylserine. Circ.Res. 77: 1136–1142.
L. Dini, A. Lentini, G. D. Diez, M. Rocha, L. Falasca, L. Serafino, and F. Vidal-Vanaclocha (1995). Phagocytosis of apoptotic bodies by liver endothelial cells. J.Cell Sci. 108: 967–973.
S. C. Finnemann and E. Rodriguez-Boulan (1999). Macrophage and retinal pigment epithelium phagocy-tosis: Apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase c regulates alphavbeta5 binding and cytoskeletal linkage. J.Exp.Med. 190: 861–874.
G. M. Walsh, D. W. Sexton, M. G. Blaylock, and C. M. Convery (1999). Resting and cytokine-stimulated human airway epithelial cells recognize and engulf apoptotic eosinophils. Blood 94: 2827–2835.
V. A. Fadok, A. de Cathelineau, D. L. Daleke, P. M. Henson, and D. L. Bratton (2001). Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phago-cytosis of apoptotic cells by macrophages and fibroblasts. J.Biol.Chem. 276: 1071–1077.
S. C. Finnemann and R. L. Silverstein (2001). Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocy-tosis by the retinal pigment epithelium. J.Exp.Med. 194: 1289–1298.
D. W. Sexton, M. G. Blaylock, and G. M. Walsh (2001). Human alveolar epithelial cells engulf apoptotic eosinophils by means of integrin-and phosphatidylserine receptor-dependent mechanisms: A process upregulated by dexam-ethasone. J.Allergy Clin.Immunol. 108: 962–969.
R. Parnaik, M. C. Raff, and J. Scholes (2000). Differences be-tween the clearance of apoptotic cells by professional and non-professional phagocytes. Curr.Biol. 19: 857–860.
W. Wood, M. Turmaine, R. Weber, V. Camp, R. A. Maki, S. R. McKercher, and P. Martin (2000). Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development 127: 5245–5252.
A. Gorska, J. Derynck, H. Moses, and R. Serra (1998). Dominant-negative interference of the transforming growth factor beta type II receptor in mammary gland epithelium results in alveolar hyperplasia and differentiation in virgin mice. Cell Growth Differ. 9: 229–238.
D. Linzer and S. Fisher (1999). The placenta and the prolactin family of hormones: Regulation of the physiology of preg-nancy. Mol.Endocrinol. 13: 837–840.
D. Grattan (2001). The actions of prolactin in the brain during pregnancy and lactation. Prog.Brain Res. 133: 153–171.
R. P. Garofalo and A. S. Goldman (1998). Cytokines, chemokines and colony-stimulating factors in human milk: The 1997 update. Biol.Neonate 74: 134–142.
J. Fata, K. Leco, E. Voura, H. Yu, P. Waterhouse, G. Murphy, R. Moorehead, and R. Khokha (2001). Accelerated apoptosis in the TIMP-3-deficient mammary gland. J.Clin.Invest. 108: 831–841.
L. R. Lund, S. Bjorn, M. Sternlicht, B. Nielsen, H. Solberg, P. Usher, R. Osterby, I. Christensen, R. Stephens, T. Bugge, K. Dano, and Z. Werb (2000). Lactational competence and involution of the mouse mammarygland require plasminogen. Development 127: 4481–4492.
R. A. Lang and J. M. Bishop (1993). Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 74: 453–462.
M. Schmitt-Ney, B. Happ, P. Hofer, N. E. Hynes, and B. Groner (1992). Mammary gland-specific nuclear factor activity is pos-itively regulated by lactogenic hormones and negatively by milk stasis. Mol.Endocrinol. 6: 1988–1997.
R. Jaggi, A. Marti, K. Guo, Z. Feng, and R. Friis (1996). Reg-ulation of physiological apoptosis: Mouse mammmary invo-lution. J.Dairy Sci. 79: 1074–1084.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Monks, J., Geske, F.J., Lehman, L. et al. Do Inflammatory Cells Participate in Mammary Gland Involution?. J Mammary Gland Biol Neoplasia 7, 163–176 (2002). https://doi.org/10.1023/A:1020351919634
Issue Date:
DOI: https://doi.org/10.1023/A:1020351919634