Abstract
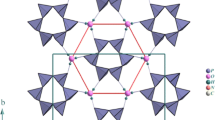
Syntheses and single-crystal X-ray structural results are reported for three new mixed diphosphates of the family AI 2BII 3(P2O7)2; Ag2Co3(P2O7)2 (I), Ag2Mn3(P2O7)2 (II), and Na2Cd3(P2O7)2 (III). All crystallize in the triclinic system, space group P1 bar: (I) a = 5.351(4), b = 6.375(4), c = 16.532(4) Å, α = 80.83(6) β = 81.45(4), γ = 72.87(5)°, V = 528.9(6) Å3, Z = 2, D calc = 4.649 mg/m3, R/Rw = 0.0428/0.0548 for 3949 obs. reflns; (II) a = 5.432(7), b = 6.619(6), c = 16.51(3) Å, α = 80.78(8) β = 82.43(9), γ = 72.82(7)°, V = 557.7(13) Å3, Z = 2, D calc = 4.338 mg/m3, R/Rw = 0.0679/0.1303 for 2100 obs. reflns and (III) a = 5.67(3), b = 7.08(4), c = 7.90(4) Å, α = 77.0(2), β = 82.5(2), γ = 67.8(2)°, V = 286(3) Å3, Z = 2, D calc = 4.249 mg/m3, R/Rw = 0.0307/0.0342 for 1945 obs. reflns. (I) and (II) are isostructural but (III) is of a different type. All three structures are characterized by layers of P2O7 groups alternating with layers of mixed metal atoms. Differences are seen in the conglomerate bonding patterns of B atoms and in the irregular geometry of Ag in (I) and (II) compared to the octahedral bonding seen for Na in (III). The differences in structure may be understood in terms of the ratios of the ionic radii of A and B atoms.
Similar content being viewed by others
References
Durif, A. Crystal Chemistry of Condensed Phosphates; Plenum Press: New York, 1995.
Elmaadi, A.; Boukhari, A.; Holt, E.M.; Flandrois, S. C.R. Acad. Sci. Paris 1994, 318, 765.
Handizi, A.; Boukhari, A.; Holt, E.M.; Aride, J.; Flandrois, S. Mat. Res. Bull. 1993, 28, 1241.
Handizi, A.; Boukhari, A.; Holt, E.M.; Aride, J.; Belaiche, M.; Drillon, M. Eur. J. Solid State Inorg. Chem. 1994; 31, 123.
Benkhouja, K.; Zahir, M.; Sadel, A.; Handizi, A.; Boukhari, A.; Holt, E.M.; Aride, J.; Drillon, M. Mat. Res. Bull. 1995, 30(1), 49.
Dridi, N.; Boukhari, A.; Reau, J.M.; Holt, E.M. Solid State Ionics 1998, 107, 25.
Dridi, N.; Boukhari, A.; Reau, J.M.; Arbib, E.; Holt, E.M. Solid State Ionics 2000, 127, 141.
Erragh, F.; Boukhari, A.; Sadel, A.; Holt, E.M. Acta Crystallogr. 1998, C54, 1373.
Bennazha, J.; Boukhari, A.; Holt, E.M. Solid State Sciences 1999, 1, 373.
Erragh, F.; Boukhari, A.; Sadel, A.; Holt, E.M. Acta Crystallogr. 1998, C54, 1746.
Amroussi, F.; Moqine, A.; Boukhari, A.; Holt E.M. Eur. J. Solid State Inorg. Chem. 1997, 34, 161.
Eddahby, L.; Berrada, A.; Boukhari, A.; Holt, E.M. J. Sol. State Inorg. Chem. 1997, 34(6), 527.
Belharouak, I.; Parent, C.; Gravereau, C.; Chaminade, J. P.; Le Flem, G.; Moine, B. J. Solid State Chem. 2000, 149, 284.
Yamada, T.; Koizumi.; H. J. Cryst. Growth 1983, 64, 558.
Gabelica-Robert, M. Proceedings of the Second European Conference on Solid State Chemistry, Elsevier: Amsterdam, 1983, p 475.
Bennazha, J.; Elmaadi, A.; Boukhari, A.; Holt, E.M. Submitted 2000.
XSCANS Users Manual; Siemens Analytical X-ray Instruments, Inc.: Madison, WI, 1991.
Sheldrick, G.M. SHELXS-97, Acta Crystallogr. 1990, A46, 467.
Sheldrick, G.M. SHELXL-98, A Program for the Refinement of Crystal Structures from Diffraction Data; University of Göttingen: Germany, 1998.
Elmarzouki, A.; Boukhari, A.; Holt, E.M. Denver Meeting, American Chemical Society, April 1993.
Averbuch-Pouchot, M.T. Z. Kristallogr. 1985, 171, 119.
Rissouli, K.; Benkhouja, K.; Sadel, A.; Bettach, M.; Zahir, M.; Giorgi, M.; Pierrot, M. Acta Crystallogr. 1996, C52, 2960.
Shannon R.D. Acta Crystallogr. 1976, A32, 751.
Brown, I. D. Struct. Bonding Cryst. 1981, 2, 1.
Averbuch-Pouchot, M.T. J. Solid State Chem. 1993, 102, 93.
Averbuch-Pouchot, M.T.; Durif, A. Eur. J. Solid State Inorg. Chem. 1992, 29, 993.
Hammond, R.; Barbier, J.; Gallardo, C. J. Solid State Chem. 1998, 141, 177.
Jost, K.H. Acta Crystallogr. 1961, 14, 779.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bennazha, J., Erragh, F., Boukhari, A. et al. Identification of a new family of diphosphate compounds, AI2BII3(P2O7)2: Structures of Ag2Co3(P2O7)2, Ag2Mn3(P2O7)2, and Na2Cd3(P2O7)2 . Journal of Chemical Crystallography 30, 705–716 (2000). https://doi.org/10.1023/A:1012294026877
Issue Date:
DOI: https://doi.org/10.1023/A:1012294026877