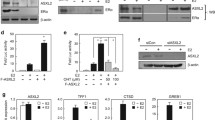
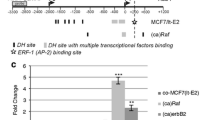
Abstract
DNA methylation is an epigenetic modification that is associated with transcriptional silencing of gene expression in mammalian cells. Hypermethylation of the promoter CpG islands contributes to the loss of gene function of several tumor related genes, including estrogen receptor α (ER) and progesterone receptor (PR). Gene expression patterns are also heavily influenced by changes in chromatin structure during transcription. Indeed both the predominant mammalian DNA methyltransferase (DNMT1), and the histone deacetylases (HDACs) play crucial roles in maintaining transcriptionally repressive chromatin by forming suppressive complexes at replication foci. These new findings suggest that epigenetic changes might play a crucial role in gene inactivation in breast cancer. Further, inhibition of DNA methylation and histone deacetylation might be a therapeutic strategy in breast cancer, especially for those cancers with ER and PR negative phenotypes.
Similar content being viewed by others
REFERENCES
M. Ehrlich, M. A. Gama-Sosa, L. H. Huang, R. M. Midgett, K. C. Kuo, R. A. McCune, and C. Gehrke (1982). Amount and distribution of 5-methylcytosine in human DNA from different types of cells. Nucleic Acids Res. 10:2709–2721.
A. P. Bird (1986). CpG-rich islands and the function of DNA methylation. Nature 321:209–213.
S. U. Kass, D. Pruss, and A. P. Wolffe (1997). How does DNA methylation repress transcription? Trends Genet. 13:444–449.
M. S. Bartolomei and S. M. Tilghman (1997). Genomic imprinting in mammals. Ann. Rev. Genet. 31:493–525.
R. Jaenisch, C. Beard, J. Lee, Y. Marahrens, and B. Panning (1998). Mammalian X chromosome inactivation. Novartis Foundation Symp. 214:200–209.
S. B. Baylin and J. G. Herman (2000). DNA hypermethylation in tumorigenesis. Trends Genet. 16:168–174.
M. S. Tucker and T. H. Bestor (1997). Formation of methylation patterns in mammalian genome. Mutation Res. 386:119–130.
E. Li., T. H. Bestor, and R. Jaenisch (1992). Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69:615–926.
Y. Liu, E. J. Oakeley, L. Sun, and J. P. Jost (1998). Multiple domains are involved in the targeting of the mouse DNA methyltransferase in the DNA replication foci. Nucleic Acids Res. 26:1038–1045.
I. Rhee, K W. Jair, R. W. C. Yen, C. Lengauer, J. G. Herman, K. W. Kinzler, B. Vogelstein, S. B. Baylin and K. E. Schuebel (2000). CpG methylation is maintained in human cancer cells lacking DNMT1. Nature 404:1003–1007.
J. A. Yoder and T. H. Bestor (1998). A candidate mammalian DNA methyltransferase related to pmt1p of fission yeast. Humar Mol. Genet. 7:279–284.
M. Okano, S. Xie, and E. Li (1998). Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res. 26:2536–2540.
V. Wyngaert, J. Sprengel, S. U. Kass, and W. H. Luyten (1998). Cloning and analysis of a novel human putative DNA methyltransferase. FEBS Lett. 426:283–289.
M. Okano, S. Xie, and E. Li (1998). Cloning and characterization of a family of novel mammalian DNA(cytosine-5) methyltransferases. Nature Genet. 19:219–220.
S. Xie, Z. Wang, M. Okano, M. Nogami, Y. Li, W. W. He, K. Okumura, and E. Li (1999). Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene 236:87–95.
S. E. Goelz, B. Vogelstein, S. R. Hamilton, and A. P. Feinberg (1985). Hypomethylation of DNA from benign and malignant human colon neoplasms. Science 228:187–190.
L. Momparler and V. Bovenzi (2000). DNA methylation and cancer. J. Cell. Physiol. 183:145–154.
S. A. Belinsky, K. J. Nikula, W. A. Palmisano, R. Michels, G. Saccomanno, E. Gabrielson, S. B. Baylin, and J. G. Herman (1998). Aberrant methylation of p16INK4a is an early event in lung cancer and a potential biomarker for early diagnosis. Proc. Natl. Acad. Sci. U.S.A. 95:11891–11896.
D. J. Wong, S. A. Foster, D. A. Galloway, and B. J. Reid (1999). Progressive region-specific de novo methylation of the p16CpG island in primary human mammary epithelial cell strains during escape from M(0) growth arrest. Mol. Cell. Biol. 19:5642–5651.
G. J. Nuovo, T. W. Plaia, S. A. Belinsky, S. B. Baylin, and J.G. Herman (1999). In situ detection of the hypermethylationinduced inactivation of the p16 gene as an early event in oncogenesis. Proc. Natl. Acad. Sci. U.S.A. 96:12754–12759.
T. Kiyono, S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz (1998). Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84–88.
J. G. Herman, A. Umar, K. Polyak, J. R. Graff, N. Ahuja, J. P. Issa, S. Markowitz, J. K. Wilson, S. R. Hamilton, K.W. Kinzler, M. F. Kane, R. D. Kolodner, B. Vogelstein, T. A. Kunkel, and S. B. Baylin (1998). Incidence and functional consequences of hMLH1 promoter hpermethylation in colorectal carcinoma. Proc. Natl. Acad. Sci. U.S.A. 95:6870–6875.
M. Esteller, L. Catasus, X. Matias-Guiux, G. L. Mutter, J. Prat, S. B. Baylin and J. G. Herman (1999). HMLH1 promoter hypermethylation is an early event in human endometrial tumorigenesis. Amer. J. Pathol. 155:1767–1772.
W. H. Lee, R. A. Morton, J. I. Epstein, J. D. Brooks, P. A. Campbell, G. S. Bova, W. S. Hsieh, W. B. Issacs, and W. G. Nelson (1994). Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 91:11733–11737.
M. Esteller, P. G. Corn, J. M. Urena, E. Gabrielson, S. B. Baylin, and J. G. Herman (1998). Inactivation of glutathione S-transferase P1 gene by promoter hypermethylation in human neoplasia. Cancer Res. 58:4515–4518.
M. Grunstein (1997). Histone acetylation in chromatin structure and transcription. Nature 389:349–352.
C. A. Mizzen and C.D. Allis (1998). Linking histone acetylation to transcriptional regulation. Cell Mol. Life Sci. 54:6–20.
C. A. Hassig, J. K. Tong, T. C. Fleischer, T. Owa, P. G. Grable, D. E. Ayer, and S. L. Schreiber (1998). A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc. Natl. Acad. Sci. U.S.A. 95:3519–3524.
U. H. Weidle and A. Grossmann (2000). Inhibition of histone deacetylases: Anew strategy to target epigenetic modifications for anticancer treatment. Anticancer Res. 20:1471–1486.
T. Taki, M. Sako, M. Tsuchida, and Y. Hayashi (1997). The t(11;16) (q23;p13) translocation in myelodysplastic syndrome fuses the MLL gene to the CBP gene. Blood 89:3945–3950.
O. M. Sobulo, J. Borrow, R. Tomek, S. Reshmi, A. Harden, B. Schlegelberger, D. Housman, N. A. Doggett, J. D. Rowley, and N. J. Zeleznik-Le (1997). MLLis fused to CBP, a histone acetyltranferase, in therapy-related acute myeloid leukemia with a t(11;16) (q23;p13.3). Proc. Natl. Acad. Sci. U.S.A. 94:8732–8737.
R. I. Yarden and L. C. Brody (1999). BRCA1 interacts with components of the histone deacetylase complex. Proc. Natl. Acad. Sci. U.S.A. 96:4983–4988.
I. Irminger-Finger, B. D. Siegel, and W. C. Leung (1999). The functions of breast cancer susceptibility gene 1 (BRCA1) and its associated proteins. Biol. Chem. 380:117–128.
H. Siddique, J. P. Zou, V. N. Rao, and E. S. P. Reddy (1998). The BRCA2 is a histone acetyltransferase. Oncogene 16:2283–2285.
L.M. Mielnicki, H. L. Asch, and B.B. Asch(2001). Genes, Chromatin, and breast cancer: An epigenetic tale. J. Mam. Gland Biol. Neoplasia, 6(2).
T. H. Bestor (1998). Methylation meets acetylation. Nature 393:311–312.
S. Eden, T. Hashimshony, I. Keshet, H. Cedar, and A.W. Thorne (1998). DNA methylation models histone acetylation. Nature 394:842.
M. R. Rountree, K. E. Bachman, and S. B. Baylin (2000). DNMT1 binds HDAC2 and a new co-represor, DNMAP1, to form a complex at replication foci. Nat. Genet. 25:269–277.
K. D. Robertson, S. Ait-Si-Ali, T. Yokochi, P. A. Wade, P. L. Jones, and A. P. Wolffe (2000). DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2Fresponsive promoters. Nat. Genet. 25:338–342.
R.G. Lapidus, S. J. Nass, and N. E. Davidson (1998). The loss of estrogen and progesterone receptor gene expression in human breast cancer. J. Mam. Gland Biol. Neoplasia 3:85–94.
D. L. Ricketts, G. Turnbull, R. Ryall, N. S. B. Baskshi, N. S. B. Raswon, J-C. Gazet, C. Nolan, and R. C. Coombes (1991). Estrogen and progesterone receptor in the normal female breast. Cancer Res. 51:1817–1822.
O. W. Peterson, P. E. Hoyer, and B. Van Deur (1987). Frequency and distribution of estrogen positive cells in normal, non-lactating human breast tissue. Cancer Res. 47:5748–5751.
W. L. McGuire (1978). Hormone receptors: Their role in predicting prognosis and response to endocrine therapy. Seminar Oncol. 5:428–433.
T. Kuukasjarvi, J. Kononen, K. Helin, K. Holli, and J. Isola (1996). Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J. Clin. Oncol. 14:25284–25289.
G. L. Greene, P. Hilna, M. Waterfield, A. Baker, Y. Hort, and J. Shine (1986). Sequence and expression of human estrogen receptor complementary DNA. Science 231:1150–1154.
S. Green, P. Walter, V. Kumar, A. Krust, P-M. Bornet, P. Argos, and P. Chambon (1986). Human oestrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nature 320:134–139.
M. Ponglikitmonkol, S. Green, and P. Chambon (1988). Genomic organization of the human oestrogen receptor gene. EMBO J. 7:3385–3388.
R. Piva, R. Gambari, F. Zorzato, L. Kumar, and L. del Senno (1992). Analysis of upstream sequences of the human estrogen receptor gene. Biochem. Biophys. Res. Commun. 183:996–1002.
R. J. Weigel and E. C. de Coninck (1993). Transcriptional control of estrogen receptor in estrogen receptor-negative breast carcinoma. Cancer Res. 53:3472–3474.
Y. L. Ottaviano, J-P. Issa, F. F. Parl, H. S. Smith, S. B. Baylin, and N. E. Davidson (1994). Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 54:2552–2555.
S. J. Nass, A. T. Ferguson, D. El-Ashry, W. G. Nelson, and N. E. Davidson (1999). Expression of DNA methyltransferase (DNMT) and the cell cycle in human breast cancer cells. Oncogene 18:7453–7461.
R. G. Lapidus, A. T. Ferguson, Y. L. Ottaviano, F. F. Parl, S. B. Baylin, J-P. J. Issa, and N. E. Davidson (1996). Methylation of estrogen and progesterone receptor gene 50 CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin. Cancer Res. 2:805–810.
R.G. Lapidus, S. J. Nass, K.A. Butash, F.F. Parl, S. A. Weitzman, J. G. Graff, J. G. Herman, and N. E. Davidson (1998). Mapping of ER gene CpG island methylation by methylation-specific polymerase chain reaction. Cancer Res. 58:2515–2519.
H. Iwase, Y. Omoto, H. Iwata, T. Toyama, Y. Hara, Y. Ando, Y. Ito, Y. Fujii, and S. Kobayashi (1999). DNAmethylation analysis at distal and proximal promoter regions of the oestrogen receptor gene in breast cancers. Brit. J. Cancer 80:1982–1986.
A. T. Ferguson, R.G. Lapidus, S. B. Baylin, and N. E. Davidson (1995). Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 55:2279–2283.
L. D. Read, C. E. Snider, J. S. Miller, G. L. Greene, and B. S. Katzenellenbogen. (1988). Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol. Endocrinol. 2:263–271.
A. T. Ferguson, R. G. Lapidus and N. E. Davidson (1998). Demethylation of the progesterone receptor CpG island is not required for progesterone receptor gene expression. Oncogene 17:577–583.
E. E. Cameron, K. E. Bachman, S. Myohanen, J. G. Herman, and S. B. Baylin (1999). Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 21:103.
X. Nan, H. H. Ng, C. A. Johnson, C. D. Laherty, B. M. Turner, T. N. Eisenman, and A. Bird (1998). Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386–389.
P. L. Jones, G. J. Veenstra, P. A. Wade, D. Vermaak, S. U. Kass, N. Landsberger, J. Strouboulis, and A. P. Woffe (1998). Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19:187–189.
R. J. Lin, L. Nagy, S. Inoue, W. Shao, W. H. Jr. Miller, and R. M. Evans (1998). Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391:811–814.
F. Grignani, S. DeMatteis, C. Nervi, L. Tomassoni, V. Gelmetti, M. Cioce, M. Fanelli, M. Ruthardt, F. F. Ferrara, I. Zamir, C. Seiser, F. Grignani, M. A. Lazar, S. Minucci, and P. G. Pellicci (1998). Fusion proteins of the retinoic acid receptor-? recruit histone deacetylase in promyelocytic leukaemia. Nature 391:815–818.
R. P. Warrell, Jr., L. Z. He, V. Richon, E. Calleja, and P. P. Pandolfi (1998). Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J. Natl. Cancer Inst. 90:1621–1625.
X. Yang, A. T. Ferguson, S. J. Nass, D. L. Philips, K. A. Butash, S.M. Wang, J.G. Herman, and N. E. Davidson (2000). Transcriptional activation of estrogen receptor ? in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 60:6890–6894.
S. Charache, G. Dover, K. Smith, C. C. Talbot, Jr., M. Moyer, and S. Boyer (1983). Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation around the ?-?-? globin gene complex. Proc. Natl. Acad. Sci. U.S.A. 80:4842–4846.
T. J. Ley, J. DeSimone, N. P. Anagnou, G. H. Keller, R. K. Humphries, P. H. Turner, N. S. Young, P. Keller, and A. W. Nienhuis (1982). 5-azacytidine selectively increases ?-globin synthesis in a patient with ?-thalassemia. N. Engl. J. Med. 307:1469–1475.
G. J. Dover, S. Charache, S. H. Boyer, G. Vogelsang, and M. Moyer (1985). 5-azacytidine increased HbF production and reduces anemia in sickle cell disease. Dose-response analysis of subcutaneous and oral dosing regimens. Blood 66:527–532.
A. R. MacLeod and M. Szyf (1995). Expression of antisense to DNA methyltransferase mRNA induces DNA demethylation and inhibits tumorigenesis. J. Biol. Chem. 270:8037–8043.
S. Ramchandani, A. R. MacLeod, M. Pinard, E. von Hofe, and M. Szyf (1997). Inhibition of tumorigenesis by a cytosine-DNA, methyltransferase, antisense oligonucleotide. Proc. Natl. Acad. Sci. U.S.A. 94:684–690.
B. G. Heerdt, M. A. Houston, G. M. Anthony, and L. H. Augenlicht (1999). Initiation of growth arrest and apoptosis of MCF-7 mammary carcinoma cells by tributyrin, a triglyceride analogue of the short-chain fatty acid butyrate, is associated with mitochondrial activity. Cancer Res. 59:1584–1591.
M. A. Carducci, J. B. Nelson, K. M. Chan-Tack, S. R. Ayyagari, W. H. Sweatt, P. A. Campbell, W. G. Nelson, and J. W. Simons (1996). Phenylbutyrate induces apoptosis in human prostate cancer and is more potent than phenylacetate. Clin. Cancer Res. 2:379–387.
A. F. Collins, H.A. Pearson, P. Giardina, K. T. McDonagh, S.W. Brusilow, and G. J. Dover (1995). Oral sodium phenylbutyrate therapy in homozygous ?-thalassemia: A clinical trial. Blood 85:43–49.
L. A. McPherson and R. J. Weigel (1999). AP2? and AP2?: A comparison of binding site specificity and trans-activation of the estrogen receptor promoter and single site promoter constructs. Nucleic Acids Res. 27:4040–4049.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, L., Yang, X. & Davidson, N.E. Role of DNA Methylation and Histone Acetylation in Steroid Receptor Expression in Breast Cancer. J Mammary Gland Biol Neoplasia 6, 183–192 (2001). https://doi.org/10.1023/A:1011308707512
Issue Date:
DOI: https://doi.org/10.1023/A:1011308707512