Abstract
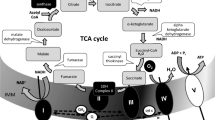
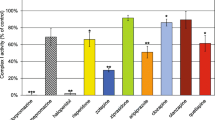
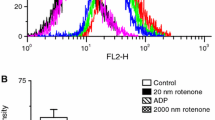
The effects of fluoxetine on the oxidative phosphorylation of mitochondria isolated from rat brain and on the kinetic properties of submitochondrial particle F1F0-ATPase were evaluated. The state 3 respiration rate supported by pyruvate + malate, succinate, or ascorbate + tetramethyl-p-phenylenediamine (TMPD) was substantially decreased by fluoxetine. The IC50 for pyruvate + malate oxidation was ∼ 0.15 mM and the pattern of inhibition was the typical one of the electron-transport inhibitors, in that the drug inhibited both ADP- and carbonyl cyanide m-chlorophenylhydrazone (CCCP)-stimulated respirations and the former inhibition was not released by the uncoupler. Fluoxetine also decreased the activity of submitochondrial particle F1F0-ATPase (IC50 ∼ 0.08 mM) even though K0.5 and activity of Triton X-100 solubilized enzyme were not changed substantially. As a consequence of these effects, fluoxetine decreased the rate of ATP synthesis and depressed the phosphorylation potential of mitochondria. Incubation of mitochondria or submitochondrial particles with fluoxetine under the conditions of respiration or F1F0-ATPase assays, respectively, caused a dose-dependent enhancement of 1-anilino-8-naphthalene sulfonate (ANS) fluorescence. These results show that fluoxetine indirectly and nonspecifically affects electron transport and F1F0)-ATPase activity inhibiting oxidative phosphorylation in isolated rat brain mitochondria. They suggest, in addition, that these effects are mediated by the drug interference with the physical state of lipid bilayer of inner mitochondrial membrane.
Similar content being viewed by others
References
Sommi RW, Crismon ML, Bowden CL: Fluoxetine: A serotoninspecific second generation antidepressant. Pharmacotherapy 7: 1–15, 1987
Henry JA: Toxicity of antidepressants: Comparisons with fluoxetine. Int J Clin Psychopharmacol 6(suppl): 22–27, 1992
Stokes PE: Fluoxetine: A five-year review. Clin Ther 15: 216–243, 1993
Messina FS: Fluoxetine: Adverse effects and drug-drug interactions. Clin Toxicol 31: 603–630, 1993
Parker WB, Cheng Y: Disruption of energy metabolism and mitochondrial function. In: L.W. Chang, W. Slikker (eds). Neurotoxicology: Approaches and Methods. Academic Press Inc., 1995, pp 483–490
Ramsay RR, Singer TP: Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem 261: 7585–7587, 1986
Caccia S, Cappi M, Fracasso C, Garattini S: Influence of dose and route of administration on the kinetics of fluoxetine and its metabolite norfluoxetine in the rat. Psychopharmacol 100: 509–514, 1990
Brand MD: The proton leak across mitochondrial inner membrane. Biochim Biophys Acta 1018: 128–133, 1990
Hatefi Y. ATP synthesis in mitochondria. Eur J Biochem 218: 759–767, 1993
Pedersen PL: Frontiers in ATP synthase research: Understanding the relationship between subunit movements and ATP synthesis. J Bioenerg Biomemb 28: 389–395, 1996
Souza MEJ, Polizello ACM, Uyemura SA, Castro-Silva O Jr, Curti C: Effect of fluoxetine on rat liver mitochondria. Biochem Pharmacol 48: 535–541, 1994
Uyemura SA, Curti C: Steady-state kinetic properties of F0F1-ATPase: The pH effect. Int J Biochem 24: 1743–1748, 1992
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976
Chance B, Williams GR: Respiration enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 217: 409–427, 1956
Cain K, Skilleter DN: Preparation and use of mitochondria in toxicological research. In: K. Snell, B. Mullock (eds). Biochemical Toxicology – A Practical Approach. IRL Press, Oxford, 1987, pp 217–254
Lemasters JJ, Hackenbrock CR: Continuous measurement and rapid kinetics of ATP synthesis in rat liver mitochondria, mitoplasts and inner membrane vesicles determined by firefly-luciferase luminescence. Eur J Biochem 67: 1–10, 1976
Slater EC, Rosing J, Mol A: The phosphorylation potential generated by respiring mitochondria. Biochim Biophys Acta 292: 534–553, 1973
Heinonen JK, Lahti RJ: A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113: 313–317, 1981
Leone FA, Degreve L, Baranauskas JA: SIGRAF: A versatile computer program for fitting enzyme kinetic data. Biochem Educ 20: 94–96, 1992
Moreland DE: Effects of toxicants on oxidative phosphorylation and photophosphorylation. In: E. Hodgson, P.E. Levi (eds). Introduction to Biochemical Toxicological. Appleton & Lange, Norwalk, 1994, pp 345–366
Mingatto FE, Santos AC, Uyemura SA, Jordani MC, Curti C: In vitro interactions of nonsteroidal anti-inflammatory drugs on oxidative phosphorylation of rat kidney mitochondria: Respiration and ATP synthesis. Arch Biochem Biophys 334: 303–308, 1996
Rottenberg H: Uncoupling of oxidative phosphorylation in rat liver mitochondria by general anesthetics. Proc Natl Acad Sci USA 80: 33–13, 1983
Weinbach EC, Costa JL, Nelson BD, Glaggett CE, Hundal T, Bradley D, Morris SJ: Effects of tricyclic antidepressant drugs on energy-linked reactions in mitochondria. Biochem Pharmacol 35: 1445–1451, 1986
Hoch FL: Cardiolipins and biomembrane function. Biochim Biophys Acta 1113: 71–133, 1992
Dabbeni-Sala F, Palatini P: Mechanism of local anesthetic effect. Involvement of F0 in the inhibition of mitochondrial ATP synthase by phenothiazines. Biochim Biophys Acta 1015: 248–252, 1990
Seeman P: Anti-schizophrenic drugs – membrane receptor sites of action. Biochem Pharmacol 26: 1741–1748, 1977
Bachamann E, Zbinden G: Effect of antidepressant and neuroleptic drugs on respiratory function of rat heart mitochondria. Biochem Pharmacol 28: 3519–3524, 1979
Dhalla NS, Lee SL, Takeo S, Panagia V, Bhayana V: Effects of chlorpromazine and imipramine on rat heart subcellular membranes. Biochem Pharmacol 29: 629–633, 1980
Daum G: Lipids of mitochondria. Biochim Biophys Acta 822: 1–42, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Curti, C., Mingattao, F.E., Polizello, A.C. et al. Fluoxetine interacts with the lipid bilayer of the inner membrane in isolated rat brain mitochondria, inhibiting electron transport and F1F0-ATPase activity. Mol Cell Biochem 199, 103–109 (1999). https://doi.org/10.1023/A:1006912010550
Issue Date:
DOI: https://doi.org/10.1023/A:1006912010550