Abstract
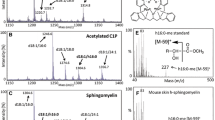
We have investigated the presence of neutral sphingomyelinases present in rabbit skeletal muscle fractions. Neutral sphingomyelinase activity measurements and immunoblot analysis of various skeletal muscle fractions indicated that most of the neutral sphingomyelinase was associated with the junctional transverse tubules. Activity gel analysis of the detergent solubilized transverse tubule fraction revealed two distinct bands corresponding to molecular weight on the order of approximately 92 and 53 kDa. Moreover, monospecific antibody raised against pure neutral sphingomyelinase recognized both the 53 and the 92 kDa protein. Peptide mapping studies revealed that both neutral sphingomyelinase isoforms were similar. Moreover, both the enzymes catalyzed the hydrolysis of sphingomyelin to phosphocholine and ceramide. Lithium stimulated and Cu2+ inhibited the activity of both of the enzyme isoforms. However, the 53 kDa isoform was insensitive to activation by Mg2+, and thus differed from the 92 kDa isoform of neutral sphingomyelinase. The localization of neutral sphingomyelinase in skeletal muscle transverse tubule membrane is consistent with transverse tubule production of the sphingomyelin-derived second messenger, sphingosine. Since sphingosine has been shown to modulate calcium release from sarcoplasmic reticulum membranes (Sabbadini et al. (1992) J Biol Chem 207: 15473-15684), our work suggests that neutral sphingomyelinase/sphingosine signaling system may be a physiologically relevant regulator of calcium levels in skeletal muscle.
Similar content being viewed by others
References
Spence MW, Callahan JN: Sphingomyelin cholesterol lipidoses: The Niewmann‐Pick Group of Diseases. In: C.R. Schriver, A.C. Beaudet, W.J. Sly, D. Valle (eds). The Metabolic Basis of Inherited Disease, 6th Ed, McGraw‐Hill, New York, 1989, Vol. II, Chapter 66, pp. 1655–1676
Chatterjee S: Neutral Sphingomyelinase. Adv Lipid Res 26: 25–48, 1993
Chatterjee S: Neutral sphingomyelinase action induces signal transduction of tumor necrosis factor‐cc in increasing cholesteryl ester synthesis in human fibroblasts. J Biol Chem 269: 879–882, 1994
Okazaki T, Bell RM, Hannun Y: Sphingomyelin turnover induced by vitamin D3 in HL‐60 cells. Role in cell differentiation. J Biol Chem 264: 19076–19080, 1989
Murray DK, Rohmann‐Wennhold A, Nelson DH: Dexamethasone effect on the phospholipid content of isolated fat cell ghosts from adrenalectomized rats. Endocrinology (Baltimore) 105: 774–777, 1979
Obeid LM, Linardic CM, Karolek LA, Hannun Y: Programmed cell death induced by ceramide. Science 258: 1769–1771, 1993
Kolesnick RN: Ceramide: A novel second messenger. Trends Cell Biol 2: 232–236, 1992
Kim MY, Linardic C, Obeid L, Hannun Y: Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor‐α ?and γ‐interferon. Special role in cell differentiation. J Biol Chem 266: 484–489, 1991
Chatterjee S: Neutral sphingomyelinase stimulates human plasma low density lipoprotein receptor activity in human fibroblasts. J Biol Chem 268: 3401–3406, 1993
Stopeck AT, Nicholson AC, Mancini FP, Hajjar DP: Cytokine regulation of low density lipoprotein receptor gene transcription in HepG2 cells. J Biol Chem 268: 17489–17494, 1993
Hannun YA, Bell RM: The sphingomyelin cycle: A prototypic sphingolipid signaling pathway. Adv Lipid Res 25: 27–41, 1993
Hannun YA, Loomis CR, Merrill AH Jr, Bell RM: Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem 261: 12604–12609, 1986
Merrill AH Jr, Sereni AM, Stevens VL, Hannun YA, Bell RM, Kinkade JM Jr: Inhibitor of phorbol ester dependent differentiation of human promyelocytic leukemic HL‐60 cells by sphinganine and other long chain bases. J Biol Chem 261: 12610–12615, 1986
Jefferson AB, Schulman H: Sphingosine inhibits calmodulin‐dependent enzymes. J Biol Chem 263: 15241–15244, 1988
Ghosh TK, Bian J, Gill DL Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science 248: 1653–1656, 1990
Sabbadini RA, Betto R, Teresi A, Cassano GF, Salvaiati G: The effects of sphingosine on sarcoplasmic reticulum membrane calcium release. J Biol Chem 267: 15474–15484, 1992
Saito A, Seiler S, Chu A, Fleischer S: Preparation and morphology of sarcoplasmic reticulurn terminal cistemae from rabbit skeletal muscle. J Cell Biol 99: 875–885, 1984
Horgan DJ, Kuypers R: Isolation of transverse tubules by fractionation of sarcoplasmic reticulurn preparations in ion‐free sucrose density gradients. Arch Biochem Biophys 253: 377–387, 1987
Luise M, Presotto C, Senter L, Betto R, Ceoldo S, Furlan S, Salvatori S, Sabbadini R, Salviati G: Dystrophin is phosphorylated by endogenous protein kinases. Biochem J 293: 243–247, 1993
Chatterjee S, Ghosh N: Neutral sphingomyelinase from human urine: Purification and preparation of monospecific antibodies. J Biol Chem 264: 12554–12561, 1989
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970
Chatterjee S, Ghosh N, Khurana S: Purification and partial characterization of uridine diphosphate galactose: Glucosylceramide, β1–> 4 galactosyltransferase (GalT‐2) from human kidney. J Biol Chem 267: 7148–7153, 1992
Slife CW, Wang E, Hunter R, Wang S, Burgess C, Liotta DC, Merrill AH Jr: Free sphingosine formation from endogenous substrates by a liver plasma membrane system with a divalent cation dependence and a neutral pH optimum. J Biol Chem 264: 10371–10377, 1989
Mathias S, Dressler KA, Kolesnick RN: Characterization of a ceramide‐activated protein kinase: Stimulation by tumor necrosis factor alpha. Proc Nad Acad Sci USA. 88: 10009–10013, 1991
Zhang H, Buckley NE, Gibson K, Spiegel S: Sphingosine stimulates cellular proliferation via a protein kinase C‐independent pathway. J Biol Chem 265: 76–81, 1990
Merrill AH Jr, Jones DD: An update of the enzymology and regulation of sphingomyelin metabolism. Biochem Biophys Acta 1044: 1–12, 1990
Sabbadini R, McNutt W, Jenkins G, Betto R, Salviati G: Sphingosine is endogenous to cardiac and skeletal muscle. Biochem Biophys Res Comm 193: 752–758, 1993
Salvatori S, Furlan S, Milkikan B, Sabbadini R, Betto R, Margreth A, Salviati G: Localization of protein kinase C in skeletal muscle T‐tubule membranes. Biochem Biophys Res Comm 196: 1073–1080, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghosh, N., Sabbadini, R. & Chatterjee, S. Identification, partial purification, and localization of a neutral sphingomyelinase in rabbit skeletal muscle: Neutral sphingomyelinase in skeletal muscle. Mol Cell Biochem 189, 161–168 (1998). https://doi.org/10.1023/A:1006910200656
Issue Date:
DOI: https://doi.org/10.1023/A:1006910200656