Abstract
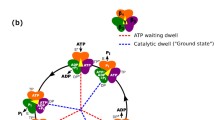
The structural organization and overall dimensions of the Escherichia coli F1-ATPase in solutionhas been analyzed by synchroton X-ray scattering. Using an independent ab initio approach,the low-resolution shape of the hydrated enzyme was determined at 3.2 nm resolution. Theshape permitted unequivocal identification of the volume occupied by the α3 β3 γ complex ofthe atomic model of the ECF1-ATPase. The position of the ^σ and ∈ subunits were found byinteractive fitting of the solution scattering data and by cross-linking studies. Laser-inducedcovalent incorporation of 2-azido-ATP established a direct relationship between nucleotidebinding affinity and the different interactions between the stalk subunits γ and ∈ with the threecatalytic subunits (β) of the F1-ATPase. Mutants of the ECF1-ATPase with the introductionof Trp-for-Tyr replacement in the catalytic site of the complex made it possible to monitorthe activated state for ATP synthesis (ATP conformation) in which the γ and ∈ subunits arein close proximity to the α subunits and the ADP conformation, with the stalk subunits arelinked to the β subunit.
Similar content being viewed by others
REFERENCES
Abrahams, J. P., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994). Nature (London) 370, 621–628.
Aggeler, R., Haughton, M. A., and Capaldi, R. A. (1995). J. Biol. Chem. 270, 9185–9191.
Aggeler R., Ogilvie I., and Capaldi R. A. (1997). J. Biol. Chem. 272, 19621–19624.
Bianchet, M. A, Hullihen, J. Pedersen, P. L., and Amzel, M. L. (1998). Proc. Natl. Acad. Sci. USA 95, 11065–11070.
Birkenhäger, R. Hoppert, M., Deckers-Hebestreit, G., Mayer, F., and Altendorf, K. (1995). Eur. J. Biochem. 230, 58–67.
Boyer, P. D. (1997). Annu. Rev. Biochem. 66, 717–749.
Bragg, P.D. and Hou, C. (1986). Biochim. Biophys. Acta. 851, 385–394
Bulygin, V. V., Duncan, T. M., and Cross, R. L. (1998). J. Biol. Chem. 273, 31765–31769.
Capaldi, R. A., Aggeler, R., Wilkens, S., and Grüber, G. (1996). J. Bioenerg. Biomembr. 28, 397–401.
Creighton, T. E. (1984). Methods Enzymol. 107, 305–329.
Cross, R. L. (1988). J. Bioener. Biomembr. 20, 395–405.
Dallmann, H. G., Flynn, T.G., and Dunn, S.D. (1992). J. Biol. Chem. 270, 24609–24614.
Dimroth, P. (1997). Biochim. Biophys. Acta 1318, 11–51.
Engelbrecht, S., Reed, J., Pennin, F., Gautheron, C., and Junge, W. (1991). Z. Naturforsch. 46c, 759–764.
Giraud, M.-F. and Velours, J. (1994). Eur. J. Biochem. 222, 851–859.
Girvin, M. E., Rastogi, V. K., Abildgaard, F., Markley, J. L., and Fillingame, R. H. (1998). Biochemistry 37, 8817–8824.
Grüber, G. (2000). Habilitationthesis, University of Osnabrück.
Grüber, G. and Capaldi, R. A. (1996a). Biochemistry 35, 3875–3879.
Grüber, G. and Capaldi, R. A. (1996b). J. Biol. Chem. 271, 32623–32628.
Grüber, G., Hausrath, A. C., Sagermann, M., and Capaldi, R. A. (1987). FEBS Lett. 410, 165–168.
Hausrath, A. C., Grüber, G., Matthews, B. W., and Capaldi, R. A. (1999). Proc. Natl. Acad. Sci. USA 96, 13697–13702.
Junge, W., Lill, H., and Engelbrecht, S. (1997). Trends Biochem. Sci. 22, 420–423.
Kato-Yamada, Y., Noji, H., Yasuda, R., Kinosita, K. Jr., and Yoshida, M. (1998). J. Biol. Chem. 273, 19375–19377.
Kozin, M. B, Volkov, V. V., and Svergun, D. I. (1997). J. Appl. Crystallogr. 30, 811–815.
Masaike, T., Mitome, N., Noji, H., Muneyuki, E., Yasuda, R., Kinosita, J. Jr., and Yoshida, M. (2000). J. Exp. Biol. 203,1–8.
Mendel-Harting, J. and Capaldi, R. A. (1991). Biochim. Biophys. Acta. 1060, 115–125.
Pedersen, P. L. and Amzel, M. (1993). J. Biol. Chem. 268, 9937–9940.
Rastogi, V. K. and Girvin, M. E. (1999). Nature (London) 402, 263–268.
Schulenberg, B., Wellmer, F., Lill, H., Junge, W., and Engelbrecht, S. (1997). Eur. J. Biochem. 249, 131–141.
Shirakihara, Y., Leslie, A. G. W., Abrahams, J. P., Walker, J. E., Ueda, T., Sekimoto, Y., Kambara, M., Saika, K., Kagawa, Y., and Yoshida, M. (1997). Structure 5, 825–836.
Singh, S., Turina, P., Bustamante, C. J., Keller, D. J., and Capaldi, R. A. (1996). FEBS Lett. 397, 30–34.
Stock, D., Leslie, A. G. W., and Walker, J. E. (1999). Science 286, 1700–1705.
Svergun, D. I., Aldag, I., Sieck, T., Altendorf, K., Koch, M. H. J., Kane, D., Kozin, M. B., and Grüber, G. (1998a). Biophys. J. 75, 2212–2219.
Svergun, D. I., Konrad, S., Huss, M., Koch, M. H. J., Wieczorek, H., Altendorf, K., Volkov, V. V., and Grüber, G. (1998b). Biochemistry 37, 17659–17663.
Tang, C. and Capaldi, R. A. (1996). J. Biol. Chem. 271, 3018–3024.
Tozer, R. G. and Dunn, S. D. (1986). Eur. J. Biochem. 161, 513–518.
Uhlin, U., Cox, G. B., and Guss, J. M. (1997). Structure 5,1219–1230.
Walker, J. E. (1994). Curr. Opinion Struct. Bio. 4, 912–918.
Walker, J. E., Saraste, M, and Gay, N. J. (1984). Biochim. Biophys. Acta 768, 164–200.
Weber, J. and Senior, A. E. (1997). Biochim. Biophys. Acta 1319, 19–58.
Weber, J., Wilke-Mounts, S., Lee, R. S. F., Grell, E., and Senior, A. E. (1993). J. Biol. Chem. 268, 20126–20133.
Wilkens, S., Dahlquist, F. W., McIntosh, L. P., Donaldson, L. W., and Capaldi, R. A. (1995). Nature Struct. Biol. 2, 961–967.
Wilkens, S., Dunn, S. D., Chandler, J., Dahlquist, F.W., and Capaldi, R. A. (1997). Nature Struct. Biol. 4, 198–201.
Zhang, Y. and Fillingame, R. H. (1995). J. Biol. Chem. 270, 24609–24614.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grüber, G. Structural and Functional Features of the Escherichia coli F 1 -ATPase . J Bioenerg Biomembr 32, 341–346 (2000). https://doi.org/10.1023/A:1005519801891
Issue Date:
DOI: https://doi.org/10.1023/A:1005519801891