Abstract
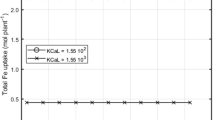
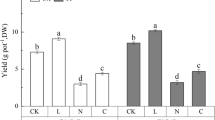
There are several studies in the literature dealing with the effect of metal-humic complexes on plant metal uptake, but none of them correlate the physicochemical properties of the complexes with agronomic results. Our study covers both aspects under various experimental conditions. A humic extract (SHE) obtained from a sapric peat was selected for preparing the metal–humic complexes used in plant experiments. Fe–, Zn– and Cu–humic complexes with a reaction stoichiometry of 2:0.25 (humic:metal, w/w) were chosen after studying their stability and solubility with respect to pH (6–9) and the humic:metal reaction stoichiometry. Wheat and alfalfa plants were greenhouse cultured in pots containing one of three model soils: an acid, sandy soil and two alkaline, calcareous soils. Treatments were: control (no additions), SHE (53 mg kg−1 of SHE), and metal (Cu, Zn and Fe)–SHE complexes (2.5 and 5 mg kg−1 of metal rate and a SHE concentration to make 53 mg kg −1). Cu- and Zn–humic complexes significantly (p≤0.05) increased the plant uptake and the DTPA-extractable soil fraction of complexed micronutrients in most plant–soil systems. However, these effects were associated with significant increases (p≤0.05) of shoot and root dry weight only in alfalfa plants. In wheat, significant increases of root and shoot dry matter were only observed in the Cu–humic treated plants growing in the acid soil, where Cu deficiency was more intense. The Fe–humic complex did not increase Fe plant assimilation in any plant–soil system, but SHE increased Fe-uptake and/or DTPA-extractable soil Fe in the wheat–calcareous soil systems. These results, taken together with those obtained from the study of the pH- and SHE:metal ratio-dependent SHE complex solubility and stability, highlight the importance of the humic:Fe complex stoichiometry on iron bioavailability as a result of its influence on complex solubility.
Similar content being viewed by others
References
Abo F and Pinta, M 1982 Influence des oligoelements sur la production de matiere seche, l'aspect morphologique et le développement du ble. CR Acad. Agric. 68, 756–765.
Alva A K and Obreza T A 1998 By-product iron-humate increases tree growth and fruit production of orange and grapefruit. Hortscience. 33, 71–74.
Barnard R O, Watt H v H van der, Dekker J, Cronje I, Mentz W H, Cillie G E B and Laker M C 1992 Application of Fe and Zn to lime-rich soils in the form of formulated coal products. Sci. Total Environ. 117/118, 569–574.
Bar-Ness E and Chen Y 1991 Manure and peat based iron-organo complexes. II. Transport in soils. Plant Soil. 130, 45–50.
Burau R G, White R G and MacGregor J M 1960 Uptake of applied iron by soybeans from calcareous soil treated with peat-based humates and synthetic chelates. Proceedings 7th International Congress of Soil Science, Madison, WI, vol 3, pp. 544–553.
Cao Y, Conklin M and Betterton E 1995 Competitive complexation of trace metals with dissolved humic acid. Environ. Health Perspect. 103, 29–32.
Chen Y and Aviad T 1990 Effects of humic substances on plant growth. In Humic Substances in Soil and Crop Sciences: Selected Readings. Ed. P MacCarthy, C E Clapp, R L Malcolm and P R Bloom. pp. 161–186. Soil Science Society of America, Inc, Madison, WI.
Chen Y 1996 Organic Matter Reactions Involving Micronutrients in Soils and their Effect on Plants. In Humic Substances in terrestrial ecosystems. Ed. A. Piccolo. pp. 507–530. Elsevier, Amsterdam.
Chen Y., Senesi N and Schnitzer M 1977 Information provided on humic substances by E4 /E6 ratios. Soil. Sci. Soc. Am. J. 41, 352–358.
Chen Y, Clapp C E, Magen H and Cline V W 1999 Stimulation of Plant Growth by Humic Substances: Effects of Iron Availability. In Understanding Humic Substances. Advances Methods, Properties and Applications. Eds. E A Ghabbour and G Davies. pp. 255–263. Royal Society of Chemistry, Cambridge.
Elprince A M 1986 Chemistry of Soil Solutions. Van Nostrand Reinhold Company, New York, USA. 411 pp.
Ennis M T 1962 Some copper-complexing properties of peat. Irish J Agric. Res. 1, 139–147.
Ennis M T and Brogan J C 1961 The availability of copper from copper-humic acid complexes. Irish J. Agric. Res. 1, 35–42.
Fitch A and Stevenson F J 1984 Comparison of models for determining stability constants of metal complexes with humic substances. Soil Sci. Soc. Am. J. 48, 1044–1050.
Gárate A., Carpena O. and Ramon A M 1984 Determinación de boro en jugos de tejidos conductores. Anal. Edaf. Agrobiol. 43, 1123–1129.
García-Mina JM 1998 Metal-humic Complexes and Plant development. PhD. thesis. University of Navarra, Pamplona, Spain.
Geering H R, Hodgson J F and Sdano C 1969 Micronutrient cation complexes in soil solution: IV. The chemical state of manganese in soil solution. Soil Sci. Soc. Am. Proc. 33, 81–85.
Gupta S K 1986 Bioverfuegbarkeit von Kupfer in einem Huminsaeure-und Klaerschlamm-komplex. Mitteilgn. Dtsch. Bodenkundl. Gesellsch. 45, 61–66.
Gupta S K and Häni H 1980 Effect of copper supplied in the form of different Cu-saturated sludge samples and copper salts on the Cu-concentration and dry matter yield of corn grown in sand. In Symposium on Copper in Animal Wastes and Sewage Sludge. Eds. P. L'Hermite and J. Dehand-schutter. pp. 67–69. Reidel Publishing Co., Dordrecht.
Hodgson J F 1969 Contribution of metal-organic complexing agents to the transport of metals to roots. Soil Sci. Soc. Am. Proc. 33, 68–75.
Hodgson J F, Geering H R and Norwell W A 1965 Micronutrient cation complexes in soil solution: I. Partition between complexed and uncomplexed forms by solvent extraction. Soil Sci. Soc. Am. Proc. 29, 665–669.
Hodgson J F, Lindsay W L and Trierweiler J F 1966 Micronutrient cation complexing in soil solution. II. Complexing of zinc and cooper in displaced solution from calcareous soils. Soil Sci. Soc. Am. Proc. 30, 723–726.
Kinniburg D, Milne CH, Benedetti M, Pinheiro J, Filius J, Koopal L and Van Riemsdijk E H 1996 Metal ion binding by humic acid: Application of the NICA-Donnan model. Environ. Sci. Technol. 30, 1687–1698.
Kumar M and Prasad B 1989 The relative contribution of natural zinc complexing agents and ZnSO4 to growth and zinc nutrition of maize. J. Nucl. Agric. Biol. 18, 29–35.
Legaz F, Serna M D, Ferrer P, Cebolla V and Primo-Millo E 1995 Nutritional Diagnosis in Citrus plants. Generalitat Valenciana, Valencia, Spain. 27 pp.
Lindsay W and Norvell WA 1978 Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 42, 421–428.
Lo S Y and Reisenauer M 1968 Zinc nutrition of alfalfa. Agron. J. 60, 464–466.
Lobartini J C and Orioli G A 1988 Absorption of iron Fe-humate in nutrient solutions by plants. Plant Soil 106, 153–157.
Loué A 1993 Oligoelements en Agriculture. Editions Natham, Paris. 577 p.
Lucena J J, Gárate A and Carpena O 1987 Iron-chelates evaluation in a calcareous soil. Plant Soil 103, 134–138.
Miravé J P and Orioli G A 1987 Edaphic mobility of complete humic acid and fractions of high and medium molecular weight. Plant Soil 104, 169–174.
Miravé J P and Orioli G A 1989 Zinc absorption and transport from complete humate and high and medium molecular weight fractions. Sci. Total Environ. 81/82, 679–682.
Miravé J P, Lobartini J C and Orioli G A 1987 Absorción y transporte de hierro a partir de fuentes orgánicas de distinto peso molecular. Ci. Suelo 5, 31–35.
Nardi S, Concheri G and Dell'Agnola G 1996 Biological activity of humus. In Humic Substances in Terrestrial Ecosystems. Ed. A. Piccolo. pp. 361–406. Elsevier, Amsterdam.
Norvell W A 1984 Comparison of chelating agents as extractants for metals in diverse soil materials. Soil Sci. Soc. Am. J. 48, 1285–1292.
O'Connor G.A, Lindsay W L and Olsen S R 1971 Diffusion of iron chelates in soil. Soil Sci. Soc. Am. Proc. 35, 407–410.
Pandeya S B, Singh A K and Dhar P 1998 Influence of fulvic acid on transport of iron in soils and uptake by paddy seedlings. Plant and Soil. 198, 117–125.
Pinton R, Cesco S, Santi S, Agnolon F and Varanini Z 1999 Water-extractable humic substances enhance iron deficiency responses by Fe-deficient cucumber plants. Plant Soil 210, 145–157.
Primo Yúfera E and Carrasco J M 1987 Química Agrícola. I. Suelos y Fertilizantes. Alhambra, Madrid. 472 pp.
Schmidt W 1999 Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 141, 1–26.
Schnitzer M, Kodama H and Ripmeester J A 1991 Determination of the aromaticity of humic substances by X-ray diffraction analysis. Soil Sci. Soc. Am. J. 55, 745–750.
Senesi N 1992 Metal-humic substance complexes in the environment. Molecular and mechanistic aspects by multiple spectroscopic approach. In Biogeochemistry of Trace Metals. Ed. C M Adriano. pp. 429–496. Lewis Publishers, New York.
Spark K M, Wells J D and Johnson B B 1997 The interaction of a humic acid with heavy metals. Ausy. J. Soil Res. 35, 89–101.
Stevenson, F.J. 1994. Humus Chemistry. Genesis, Composition, Reactions. John Wiley and Sons, Inc., New York. 496 pp.
Swift R S 1989 Molecular weight, size, shape, and charge characteristics of humic substances:some basic considerations. In Humic Substances II. In Search of Structure. Eds. M H B Hayes, P MacCarthy, R L Malcolm and R S Swift. pp. 449–466. J. Wiley, New York.
Takamatsu T and Yoshida T 1978 Determination of stability constants of metal-humic acid complexes by potentiometric titration and ion-selective electrodes. Soil Sci. 125, 377–386.
Tipping E, Fitch A and Stevenson F J 1995 Proton and copper binding by humic acid: application of a discrete-site/electrostatic ion-binding model. Eur. J. Soil Sci. 46, 95–101.
Treeby M, Marschner H and Römheld V 1989 Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial and sinthetic metal chelators. Plant and Soil 114, 217–226.
Uren N C 2001 Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In The Rhizosphere. Eds. R Pinton, Z Varanini and P Nannipieri. pp. 19–40. Marcel Dekker, Inc, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
García-Mina, J., Antolín, M. & Sanchez-Diaz, M. Metal-humic complexes and plant micronutrient uptake: a study based on different plant species cultivated in diverse soil types. Plant and Soil 258, 57–68 (2004). https://doi.org/10.1023/B:PLSO.0000016509.56780.40
Issue Date:
DOI: https://doi.org/10.1023/B:PLSO.0000016509.56780.40