Abstract
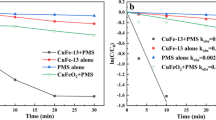
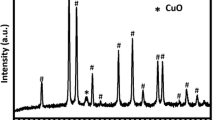
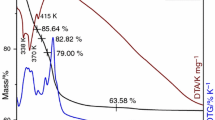
A series of Cu1−x Co x Fe2O4 (x = 0, 0.25, 0.5, 0.75, 1.0) ferrospinels prepared by low temperature coprecipitation method and glycine nitrate combustion method has been studied in gas phase methylation of phenol. Phenol methylation gives mainly o-cresol and 2,6-xylenol as major products and among various compositions, x = 0.50 shows good catalytic performance irrespective of the preparation method. The difference in properties of the fresh and spent catalysts was thoroughly characterized by adopting various physico-chemical characterization techniques with special emphasize on magnetic measurements. Various conclusions derived from magnetic study are in good agreement with our previous study of XRD and Mossbauer on same catalyst system. Redistribution of cations occurred during the reaction is evidenced from the increase of saturation magnetization in the spent. Spent x = 0.0 shows high T c close to the value of Fe3O4 indicating that the material has ended with a solid solution of Fe3O4 and CuFe2O4 along with other reduced phases.
Similar content being viewed by others
References
S.H. Oh and R.M. Sinkevitch, J. Catal. 142 (1993) 254.
H.K. Harold and C.K. Mayfair, Adv. Catal. 33 (1985) 159.
F.C. Romeijn, Philips Res. Rep. 8 (1953) 304.
Blasse, Philips Res. Rep. Suppl. 3, (1964) 96.
A. Miller, J. Appl. Phys. 30 (1959) 245.
G.M. Schwab, E. Roth, C.H. Grinzoz and N. Mavrakis, Structure and Properties of Solid Surface, eds. R. Gomer and C.S. Smith (Univ. Chicago Press, Chicago, 1953).
G.M. Schwab and A.Z. Kraut, Anorg. Allgem. Chem. 295 (1958) 36.
J.P. Suchet, Chemical Physics of Semiconductor (Van Nostrand, London, 1965).
N. Yamamoto, S. Kawano, N. Achiwa, M. Kiyama andT. Takada, Jpn. J. Appl. Phys. 12 (1973) 1830.
A.M. Sundaram and V. Sreenivasan, Phys. Status Solid A 69 (1982) K15.
M. Schmalzaried, Z. Phys. Chem. 28 (1961) 203.
R.K. Datta and R. Roy, J. Am. Ceram. Soc. 50 (1967) 578.
R.K. Datta and R. Roy, Am. Mineral 53 (1968) 1456.
C.S. Narasimhan and C.S. Swamy, Appl. Catal. 2 (1982) 315.
E. Prince, Phys. Rev. 102 (1956) 674.
G.H. Sawatzky, F. der Woude and A.H. Morrish, Phys. Rev. 187 (1969) 747.
E.J.W. Verwey and E.L. Heilmann, J. Chem. Phys. 15 (1947) 174.
B.S. Rao, T. Mathew, N.R. Shiju and R. Vetrivel, (Indian Patent pending) in-press.
B.S. Rao, K. Sreekumar and T.M. Jyothi, Indian Patent 2707/98 in-press (1998).
K. Lázár, T. Mathew, Z. Koppány, J. Megyeri, V. Samuel, S.P. Mirajkar, B.S. Rao and L. Guczi, Phys. Chem. Chem. Phys. 4 (2002) 3530.
T. Mathew, N.R. Shiju, K. Sreekumar, B.S. Rao and C.S. Gopinath, J. Catal. 210 (2002) 405.
K. Sreekumar, T. Mathew, B.M. Devassy, R. Rajgopal, R. Vetrivel and B.S. Rao, Appl. Catal. A: Gen. 205 (2001) 11.
S. Ghorpade, V.S. Darshane and S.G. Dixit, Appl. Catal. A: Gen. 166 (1998) 135.
L.A. Chick, L.R. Pederson, G.D. Maupin, J.L. Bates, L.E. Thomas and G.J. Exarhos, Mater. Lett. 10 (1990) 6.
E. Prince and R.G. Treuting, Acta. Cryst. 9 (1956) 1025.
J. Smit and H.P.J. Wijn, Advan. Electron. Electronphys. 6 (1954) 83.
R.D. Waldron, Phys. Rev. 99 (1955) 1727.
W.B. White and B.A. De Angelis, Spectrochim. Acta A 23 (1967) 985.
V.R.K. Murthy, S. Chitra Sankar, K.V. Reddy and J. Sobhanadi, Ind. J. Pure Appl. Phys. 16 (1978) 79.
M. Goodenough, Magnetism theChemicalBond (Wiley, New York, 1963).
T. Kotanigawa, M. Yamamoto, K. Shimokawa and Y. Yoshida, Bull. Chem. Soc. Jpn. 44 (1971) 1961.
J.P. Jacobs, A. Maltha, J.R.H. Reintjes, T. Drimal, V. Ponec and H.H. Brogersma, J. Catal. 147 (1994) 294.
B. Viswanathan and V.R.K. Murthy, Ferrite Materials, Science and Technology (Narosa Publishing House, 1990).
Ph. Tailhades, C. Villette, A. Rousset, G.U. Kulkarni, K.R. Kannan, C.N.R. Rao and M. Lenglet, J. Solid State Chem. 141 (1998) 56.
J. Smit and H.P.J. Wijn Ferrites, N.V. Philip's Gloeilampenfabrieken Eindhoven, The Netherlands, 1959.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathew, T., Shylesh, S., Reddy, S. et al. Redistribution of Cations Amongst Different Lattice Sites in Cu1−x Co x Fe2O4 Ferrospinels During Alkylation: Magnetic Study. Catalysis Letters 93, 155–163 (2004). https://doi.org/10.1023/B:CATL.0000017070.12868.6f
Issue Date:
DOI: https://doi.org/10.1023/B:CATL.0000017070.12868.6f