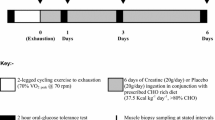
Abstract
Animal studies have shown that supra-physiological creatine monohydrate (Cr-mH) supplementation for 3 months reduced skeletal muscle creatine transporter (CRT) content. The doses of Cr-mH (1–2 g/kg/day) used in these studies were between 5 and 10 times those usually used in human studies, and it is unclear whether a down-regulation of CRT would occur in humans at the recommended doses of 0.1–0.2 g/kg/day. We measured CRT, and citrate synthase (CS) protein content using Western blotting before and after 2 months of Cr-mH supplementation and weight training in young men (N = 11 Cr-mH (0.125 g/kg/day); N = 8 placebo). CRT and CS were also measured before and after 4 months of Cr-mH supplementation and weight training in elderly (> 65 years) men and women (N = 14 Cr-mH (0.075 g/kg/day); N = 14 placebo). Finally, CRT mRNA was measured using competitive RT-PCR before and after 8–9 days of Cr-mH loading in young men and women (N = 14, CR-mH (mean = 0.18 g/kg/day); N = 13, PL). Total creatine content was significantly elevated after the Cr-mH supplementation period as compared to placebo in each of the studies. Neither Cr-mH supplementation, nor exercise training resulted in measurable alterations in CRT protein content and acute Cr-mH loading did not alter CRT mRNA. There were no gender differences in CRT mRNA or total creatine content in the young subjects and no gender differences in total creatine content or CRT protein content in the elderly subjects. Weight training in young men did not increase CS protein content, however, in the elderly there was a significant increase in CS protein content after exercise training (p < 0.05). These results demonstrated that Cr-mH supplementation during weight training resulted in increases in skeletal muscle total creatine without reductions in CRT protein and acute Cr-mH loading did not decrease CRT mRNA content.
Similar content being viewed by others
References
Sora I, Richman J, Santoro G, Wei H, Wang Y, Vanderah T, Horvath R, Nguyen M, Waite S, Roeske WR et al.: The cloning and expression of a human creatine transporter. Biochem Biophys Res Commun 204: 419-427, 1994
Iyer GS, Krahe R, Goodwin LA, Doggett NA, Siciliano MJ, Funanage VL, Proujansky R: Identification of a testis-expressed creatine transporter gene at 16p11.2 and confirmation of the X-linked locus to Xq28. Genomics 34: 143-146, 1996
Guerrero-Ontiveros ML, Wallimann T: Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: Down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol Cell Biochem 184: 427-437, 1998
Fitch CD, Shields RP: Creatine metabolism in skeletal muscle. I. Creatine movement across muscle membranes. J Biol Chem 241: 3611-3614, 1966
Loike JD, Zalutsky DL, Kaback E, Miranda AF, Silverstein SC: Extracellular creatine regulates creatine transport in rat and human muscle cells. Proc Natl Acad Sci USA 85: 807-811, 1988
Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, Kraemer WJ, Meyer RA, Spriet LL, Tarnopolsky MA, Wagenmakers AJ, Williams MH: American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc 32: 706-717, 2000
Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL: Muscle creatine loading in men. J Appl Physiol 81: 232-237, 1996
Harris RC, Soderlund K, Hultman E: Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Colch) 83: 367-374, 1992
Greenhaff PL, Bodin K, Soderlund K, Hultman E: Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am J Physiol 266: E725-E730, 1994
Greenhaff PL, Casey A, Short AH, Harris R, Soderlund K, Hultman E: Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Clin Sci (Colch) 84: 565-571, 1993
Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P: Long-term creatine intake is beneficial to muscle performance during resistance training. J Appl Physiol 83: 2055-2063, 1997
Kreider RB, Ferreira M, Wilson M, Grindstaff P, Plisk S, Reinardy J, Cantler E, Almada AL: Effects of creatine supplementation on body composition, strength, and sprint performance. Med Sci Sports Exerc 30: 73-82, 1998
Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gomez AL, Pearson DR, Fink WJ, Kraemer WJ: Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc 31: 1147-1156, 1999
Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA: Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol 91: 1041-1047, 2001
Tarnopolsky MA, Parise G, Yardley NJ, Ballantyne CS, Olantunji S, Phillips SM: Creatine-dextrose and protein-dextrose induce similar strength gains during training. Med Sci Sports Exerc 33: 2001
Tarnopolsky MA, Parise G: Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 22: 1228-1233, 1999
Tarnopolsky MA, Parshad A, Walzel B, Schlattner U, Wallimann T: Creatine transporter and mitochondrial creatine kinase protein content in myopathies. Muscle Nerve 24: 682-688, 2001
Neubauer S, Remkes H, Spindler M, Horn M, Wiesmann F, Prestle J, Walzel B, Ertl G, Hasenfuss G, Wallimann R: Downregulation of the Na(+)-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation 100: 1847-1850, 1999
Walzel B, Speer O, Boehm E, Kristiansen S, Chan S, Clarke K, Richter EA, Wallimann T: New creatine transport assay with giant plasma-membrane vesicles and identification of distinct creatine transporter isoforms in the sarcolemma and within mitochondria. Am J Physiol, 2002 (in press)
Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE: The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol 134: 625-635, 1996
Tran TT, Dai W, Sarkar HK: Cyclosporin A inhibits creatine uptake by altering surface expression of the creatine transporter. J Biol Chem 275: 35708-35714, 2000
Dodd JR, Christie DL: Cysteine-144 in the third transmembrane domain of the creatine transporter is close to a substrate binding site. J Biol Chem, 2001
Murphy R, McConell G, Cameron-Smith D, Watt K, Ackland L, Walzel B, Wallimann T, Snow R: Creatine transporter protein content, localization, and gene expression in rat skeletal muscle. Am J Physiol Cell Physiol 280: C415-C422, 2001
Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA: Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med Sci Sports Exerc 32: 291-296, 2000
Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C: X-linked creatine-transporter gene (SLC6A8) defect: A new creatine-deficiency syndrome. Am J Hum Genet 68: 1497-1500, 2001
Stockler S, Hanefeld F: Guanidinoacetate methyltransferase deficiency: A newly recognized inborn error of creatine biosynthesis. Wien Klin Wochenschr 109: 86-88, 1997
Walter MC, Lochmuller H, Reilich P, Klopstock T, Huber R, Hartard M, Hennig M, Pongratz D, Muller-Felber W: Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study. Neurology 54: 1848-1850, 2000
Tarnopolsky M, Martin J: Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 52: 854-857, 1999
Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF: Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med 5: 347-530, 1999
Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF: Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J Neurosci 18: 156-163, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tarnopolsky, M., Parise, G., Surname, MH.F. et al. Acute and moderate-term creatine monohydrate supplementation does not affect creatine transporter mRNA or protein content in either young or elderly humans. Mol Cell Biochem 244, 159–166 (2003). https://doi.org/10.1023/A:1022447604792
Issue Date:
DOI: https://doi.org/10.1023/A:1022447604792