Abstract
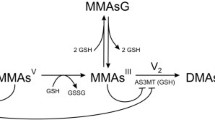
Arsenic (iAs) is a known human carcinogen and widespread contaminant in drinking water. To provide a quantitative framework for experimental design and hypothesis testing, we developed a pharmacokinetic model describing the uptake and methylation of arsenite (AsIII) in primary rat hepatocytes. Measured metabolites were inorganic As (iAs), mono-methylated As (MMA), and di-methylated As (DMA) concentration in cells and media. Transport and methylation parameters were estimated from time course data for iAs, MMA, and DMA at three initial media As(III) concentrations (0.1, 0.4, 1.0 μM). Inhibition of the formation DMA from MMA by As(III) was necessary to adequately describe the data. The data were consistent with multiple types of inhibition, although uncompetitive inhibition provided a slightly better fit. Model simulations indicate that cellular MMA (cMMA) is a key arsenical to measure; measurement of cMMA in the 4–6 hr time range using an initial concentration of 1.4 μM AsIII would provide the best experimental conditions to distinguish uncompetitive from other types of inhibition. Due to the large number of model parameters estimated from the data, we used sensitivity analysis to determine the influential parameters. Use of sensitivity surfaces facilitated the comparison of parameters over time and across doses. Predicted model responses were most sensitive to influx and efflux parameters, suggesting that transport processes are critical in determining cellular arsenical concentrations. These high sensitivities imply that independent experiments to estimate these parameters with greater certainty may be crucial for refinement of this model and to extend this model to describe methylation and transport in human hepatocytes.
Similar content being viewed by others
REFERENCES
National Research Council (NRC). Arsenic in Drinking Water. National Academy Press: Washington, D.C., 310 pp. (1999).
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for arsenic (update). ATSDR, U.S. Department of Health and Human Services, Atlanta, GA (2000).
Z. Chen, Z.-Y. Wang, and S.-J. Chen. Acute promyelocytic leukemia: cellular and molecular basis of differentiation and apoptosis. Pharmacol. Ther. 76:141-149 (1997).
E. Marafante, M. Vahter, and J. Envall. The role of the methylation in the detoxication of arsenate in the rabbit. Chem.-Biol. Interact. 56:225-238 (1985).
B. Georis, A. Cardenas, J. P. Buchet, and R. Lauwerys. Inorganic arsenic methylation by rat tissue slices. Toxicology 63:73-84 (1990).
S. M. Healy, E. A. Casarez, F. Ayala-Fierro, and H. V. Aposhian. Enzymatic methylation of arsenic compounds, V. arsenite methyltransferase activity in tissues of mice. Toxicol. Appl. Pharmacol. 148:65-70 (1998).
N. Scott, K. M. Hatelid, N. E. MacKenzie, and D. E. Carter. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem. Res. Toxicol. 6:102-106 (1993).
M. Delnomdedieu, M. M. Basti, J. D. Otvos, and D. J. Thomas. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem.-Biol. Interact. 90:139-155 (1994).
T. R. Radabaugh and H. V. Aposhian. Enzymatic reduction of arsenic compounds in mammalian systems: reduction of arsenate to arsenite by human liver arsenate reductase. Chem. Res. Toxicol. 13:26-30 (2000).
S. A. Lerman, T. W. Clarkson, and P. J. Gerson. Arsenic uptake and metabolism by liver cells is dependent on arsenic oxidation state. Chem.-Biol. Interact. 45:401-406 (1983).
W. R. Cullen, B. C. McBride, and J. Reglinski. The reaction of methylarsenicals with thiols: some biological implications. J. Inorg. Biochem. 21:179-194 (1984).
W. R. Cullen, B. C. McBride, and J. Reglinski. The reduction of trimethylarsine oxide to trimethylarsine by thiols: a mechanistic model for the biological reduction of arsenicals.J. Inorg. Biochem. 21:45-60 (1984).
E. Marafante and M. Vahter. The effect of methyltransferase inhibition on the metabolism of [74As]arsenite in mice and rabbits. Chem.-Biol. Interact. 50:49-57 (1984).
R. Zakharyan, Y. Wu, G. M. Bogdan, and H. V. Aposhian. Enzymatic methylation of arsenic compounds: assay, partial purification, and properties of arsenite methyltransferase and monomethylarsonic acid methyltransferase from rabbit liver. Chem. Res. Toxicol. 8:1029-1038 (1995).
E. Wildfang, R. A. Zakharyan, and H. V. Aposhian. Enzymatic methylation of arsenic compounds: VII. Characterization of hamster liver arsenite and methylarsonic acid methyltransferase activities in vitro. Toxicol Appl. Pharmacol. 152:366-375 (1998).
R. A. Zakharyan, F. Ayala-Fierro, W. R. Cullen, D. M. Carter, and H. V. Aposhian. Enzymatic methylation of arsenic compounds: VII. Monomethylarsonous acid (MMAIII) is the substrate for MMA methyltransferase of rabbit liver and human hepatocytes. Toxicol Appl. Pharmacol. 158:9-15 (1999).
S. Lin, Q. Shi, F. B. Nix, M. Styblo, M. A. Beck, K. M. Herbin-Davis, L. L. Hall, J. B. Simeonsson, and D. J. Thomas. A novel S-adenosyl-L-methionine: arsenic(III) methyltransferase from rat liver cytosol. J. Biol Chem, in press (2002).
M. Styblo, H. Yamauchi, and D. J. Thomas. Comparative in vitro methylation of trivalent and pentavalent arsenicals. Toxicol. Appl. Pharmacol. 135:172-178 (1995).
M. Styblo, M. Delnomdedieu, and D. J. Thomas. Mono-and dimethylation of arsenic in rat liver cytosol in vitro. Chem.-Biol. Interact. 99:147-164 (1996).
A. Gyurasics, F. Varga, and Z. Gregus. Glutathione-dependent biliary excretion of arsenic. Biochem. Pharmacol. 42:465-468 (1991).
S. V. Kala, M. W. Neely, G. Kala, C. I. Prater, D. W. Atwood, J. S. Rice, and M. W. Lieberman. The MRP2_cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J. Biol. Chem. 275:33404-33408 (2000).
Z. Gregus, A. Gyurasics, and I. Csanaky. Biliary and urinary excretion of inorganic arsenic: monomethylarsonous acid as a major biliary metabolite in rats. Toxicol. Sci. 56:18-25 (2000).
M. Vahter, R. Couch, B. Nermell, and R. Nilsson. Lack of methylation of inorganic arsenic in the chimpanzee. Toxicol. Appl. Pharmacol. 133:262-268 (1995).
M. Vahter and E. Marafante. Reduction and binding of arsenate in marmoset monkeys. Arch. Toxicol. 57:119-124 (1985).
G. M. Bogdan, A. Sampayo-Reyes, and H. V. Aposhian. Arsenic binding proteins of mammalian systems: I. Isolation of three arsenite-binding proteins of rabbit liver. Toxicology 93:175-193 (1994).
M. Styblo and D. J. Thomas. Binding of arsenicals to proteins in an in vitro methylation system. Toxicol. Appl. Pharmacol. 147:1-8 (1997).
T. Kaise, H. Yamauchi, Y. Horiguchi, T. Tani, S. Watanabe, T. Hirayama, and S. Fukui. A comparative study on acute toxicity of methylarsonic acid, dimethylarsinic acid and trimethylarsine oxide in mice. Appl. Organo. Chem. 3:273-277 (1989).
K. Yamanaka and S. Okada. Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ. Health Perspect. 102:37-40 (1994).
K. Yamanaka, K.Ohtsubo, A. Hasegawa, H. Hayashi, H. Ohji, M. Kanisawa, and S. Okada. Exposure to dimethylarsinic acid, a main metabolite of inorganic arsenics, strongly promotes tumorigenesis initiated by 4-nitroquinoline 1-oxide in the lungs of mice. Carcinogenesis 17:767-770 (1996).
S. Yamamoto, Y. Konishi, T. Matusuda, T. Murai, M.-A. Shibata, I. Matsui-Yuasa, S. Otani, K. Kuroda, G. Endo, and S. Fukushima. Cancer induction by an organic arsenic compound, dimethylarsinic acid (cacodylic acid), in F344_DuCrj rats after pretreatment with five carcinogens. Cancer Res. 55:1271-1276 (1995).
M. Wei, H. Wanibuchi, S. Yamamoto, W. Li, and S. Fukushima. Urinary bladder carcinogenicity of dimethylarsinic acid in male F344 rats. Carcinogenesis 20:1873-1876 (1999).
M. Styblo, L. Vega, D. R. Germolec, M. I. Luster, L. M. Del Razo, C. Wang, W. R. Cullen, and D. J. Thomas. Metabolism and toxicity of arsenicals in cultured cells. In Arsenic Exposure and Health Effects, (W. R. Chappell, C. O. Abernathy, and R. L. Calderon, eds.), Elsevier Science B.V., New York, pp. 311-323 (1999).
M. Styblo, L. M. Del Razo, L. Vega, D. R. Germolec, E. L. LeCluyse, G. A. Hamilton, W. Reed, C. Wang, W. R. Cullen, and D. J. Thomas. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 74:289-299 (2000).
S. Lin, L. M. Del Razo, M. Styblo, C. Wang, W. R. Cullen, and D. J. Thomas. Arsenicals inhibit thioredoxin reductase in cultured rat hepatocytes. Chem. Res. Toxicol. 14:305-311 (2001).
J. S. Petrick, F. Ayala-Fierro, W. R. Cullen, D. E. Carter, and H. V. Aposhian. Monomethylarsonous acid (MMAIII) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 163:203-207 (2000).
M. J. Mass, A. Tennant, B. C. Roop, W. R. Cullen, M. Styblo, D. J. Thomas, and A. D. Kligerman. Methylated trivalent arsenic species may be the proximate or ultimate genotoxic forms of arsenic. Chem. Res. Toxicol 14:355-361 (2001).
K. Krishnan and M. E. Andersen. Physiologically Based Pharmacokinetic Modeling in Toxicology. In: Principles and Methods in Toxicology, 3rd ed. (Hayes, A. W., ed.) Raven Press Ltd., New York, pp. 149-188 (1994).
M. V. Evans and M. E. Andersen. Sensitivity analysis and the design of gas uptake studies. Inhalation Toxicology 7:1075-1094 (1995).
E. M. Kenyon, M. Fea, M. Styblo, and M. V. Evans. Application of modeling techniques to the planning of in vitro arsenic pharmacokinetic studies. Alter. Lab. Animals 29:15-33 (2001).
K. T. Kitchin, L. M. Del Razo, J. L. Brown, W. L. Anderson, and E. M. Kenyon. An integrated pharmacokinetic and pharmacodynamic study of arsenite action. 1. Heme oxygenase induction in rats. Teratogen. Carcinogen. Mutagen. 19:385-402 (1999).
E. M. Kenyon, L. M. Del Razo, J. L. Brown, W. A. Anderson, M. F. Hughes, and K. T. Kitchin. Metabolism and disposition of arsenite (AsIII) and its relationship to heme oxygenase induction in mice. Toxicologist 48(1-S):1046.
M. Styblo, L. M. Del Razo, E. L. LeCluyse, G. A. Hamilton, C. Wang, W. R. Cullen, and D. J. Thomas. Metabolism of arsenic in primary cultures of human and rat hepatocytes. Chem. Res. Toxicol. 12:560-565 (1999).
M. Styblo, M. Delnomdedieu, and D. J. Thomas. Biological mechanisms and toxicological consequences of the methylation of arsenic. In: Toxicology of Metals-Biochemical Aspects (R. A. Goyer and M. G. Cherian, eds.), Springer Verlag, Berlin, pp. 407-433 (1995).
M. F. Hughes, M. Menache, and D. J. Thompson. Dose-dependent disposition of sodium arsenate in mice following acute oral exposure. Fund. Appl. Toxicol. 22:80-89 (1994).
E. Marafante, M. Vahter, H. Norin, J. Envall, M. Sandstrom, A. Christakopoulos, and R. Ryhage. Biotransformation of dimethylarsinic acid in mouse, hamster and man. J. Appl. Toxicol. 7:111-117 (1987).
P. O. Seglen. Preparation of isolated rat liver cells. Meth. Cell. Biol. 13:29-83 (1976).
J. P. Buchet and R. Lauwreys. Study of inorganic arsenic methylation by rat in vitro: relevance for the interpretation of observations in man. Arch. Toxicol. 57:125-129 (1985).
A. Cornish-Bowden. Fundamental of Enzyme Kinetics. Portland Press Ltd., London (1995).
J. V. Beck and K. J. Arnold. Parameter Estimation in Engineering and Science. John Wiley & Sons, New York (1977).
R. A. Yetter, F. L. Dryer, and H. Rabitz. Some interpretive aspects of elementary sensitivity gradients in combustion kinetic modeling. Combust. Flame 59:107-133 (1985).
S. Mann, P. O. Droz, and M. Vahter. A physiologically based pharmacokinetic model for arsenic exposure. I. Development in hamsters and rabbits. Toxicol. Appl. Pharmacol. 137:8-22 (1996).
S. Mann, P. O. Droz, and M. Vahter. A physiologically based pharmacokinetic model for arsenic exposure. II. Validation and application in humans. Toxicol. Appl. Pharmacol. 140:471-486 (1996).
D. Yu. A physiologically based pharmacokinetic model of inorganic arsenic. Reg. Toxicol. Pharmacol. 29:128-141 (1999).
H. V. Aposhian. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Ann. Rev. Pharmacol. and Toxicol. 37:397-419 (1997).
M. L. Gargas, H. J. Clewell, and M. E. Andersen. Gas uptake inhalation techniques and rates of metabolism of chloromethanes, chloroethanes, and chloroethylenes in the rat. Inhal. Toxicol. 2:295-319 (1990).
M. V. Evans, W. D. Crank, H.-M. Yang, and J. E. Simmons. Applications of sensitivity analysis to a physiologically based pharmacokinetic model for carbon tetrachloride in rats. Toxicol. Appl. Pharmacol. 128:36-44 (1994).
National Research Council (NRC). Science and Judgment in Risk Assessment. National Academy Press: Washington, D.C., 651pp. (1994).
Z. Wang, S. Dey, B. P. Rosen, and T. G. Rossman. Efflux-mediated resistance to arsenicals in arsenic-resistant and-hypersensitive Chinese hamster cells. Toxicol. Appl. Pharmacol. 137:112-119 (1996).
A. M. Azzarolo, G. Ritchie, and G. A. Quamme. Some characteristics of sodium-independent phosphate transport across renal basolateral membranes. Biochim. Biophys. Acta 1064:229-234 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Easterling, M.R., Styblo, M., Evans, M.V. et al. Pharmacokinetic Modeling of Arsenite Uptake and Metabolism in Hepatocytes—Mechanistic Insights and Implications for Further Experiments. J Pharmacokinet Pharmacodyn 29, 207–234 (2002). https://doi.org/10.1023/A:1020248922689
Issue Date:
DOI: https://doi.org/10.1023/A:1020248922689