Abstract
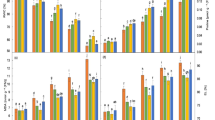
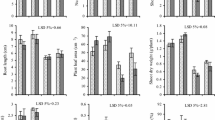
The interactive effects of salinity stress (40, 80, 120 and 160 mM NaCl) and ascorbic acid (0.6 mM), thiamin (0.3 mM) or sodium salicylate (0.6 mM) were studied in wheat (Triticum aestivum L.). The contents of cellulose, lignin of either shoots or roots, pectin of root and soluble sugars of shoots were lowered with the rise of NaCl concentration. On the other hand, the contents of hemicellulose and soluble sugars of roots, starch and soluble proteins of shoots, proline of either shoots or roots, and amino acids of roots were raised. Also, increasing NaCl concentration in the culture media increased Na+ and Ca2+ accumulation and gradually lowered K+ and Mg2+ concentration in different organs of wheat plant. Grain soaking in ascorbic acid, thiamin or sodium salicylate could counteract the adverse effects of NaCl salinity on the seedlings of wheat plant by suppression of salt stress induced accumulation of proline.
Similar content being viewed by others
References
Al-Hakimi, A.M.A.: Salinity-calcium interactions on major macromolecules and chlorophyllase activity of two green algae.-M.Sc. Thesis. Faculty of Science, Assiut University, Assiut 1995.
Ashraf, M., O'Leary, J.W.: Changes in soluble proteins in spring wheat stressed with sodium chloride.-Biol. Plant. 42: 113–117, 1999.
Bates, L.S., Waldren, R.P., Tear, I.D.: Rapid determination of free proline for water stress studies.-Plant Soil 39: 205–207, 1973.
Baur-Hoch, B., Machler, F., Nosberger, J.: Effect of carbohydrate demand on the remobilization of starch in stolons and roots of white clover (Trifolium repens L.) after defoliation.-J. exp. Bot. 41: 573–578, 1990.
Benzioni, A., Nerd, A., Rosengartner, Y., Mills, D.: Effect of NaCl salinity on growth and development of jojoba clones: I. Young plants.-J. Plant Physiol. 139: 731–736, 1992.
Boggess, S.F., Aspinall, D., Paleg, L.G.: Stress metabolism. IX. The significance of end product inhibition of proline synthesis and of compartmentation in relation to stress-induced proline accumulation.-Aust. J. Plant Physiol. 3: 513–525, 1976.
Bolarin, M.C., Santa-Cruz, A., Cayuela, E., Perez-Alfocea, F.: Short-term solute change in leaves and roots of cultivated and wild tomato seedlings under salinity.-J. Plant Physiol. 147: 463–468, 1995.
Chandler, P.M., Robertson, M.: Gene expression regulated by abscisic acid and its relation to stress tolerance.-Annu. Rev. Plant Physiology Plant mol. Biol. 45: 113–141, 1994.
Chen, Q., Liu, Y.L., Chen, Y.H.: Relationship between active oxygen damage and tonoplast H+-ATPase activity in leaves of barley seedling under salt stress.-J. Nanjing agr. Univ. 21: 21–25, 1998.
Chen, Q., Zhang, W.H., Liu, Y.L.: Effect of NaCl, glutathione and ascorbic acid on function of tonoplast vesicles isolated from barley leaves.-J. Plant Physiol. 155: 685–690, 1999.
Chen, W.S., Liu, H.Y., Liu, Z.H., Yang, L., Chen W.H.: Gibberellin and temperature influence carbohydrate content and flowering in Phalaenopsis.-Physiol. Plant. 90: 391–395, 1994.
Choudhury, N.K., Cho, H.T., Huffaker, R.C.: Ascorbate induced zeaxanthin formation in wheat leaves and photoprotection of pigment and photochemical activities during aging of chloroplasts in light.-J. Plant Physiol. 141: 551–556, 1993.
Cusido, R.M., Palazon, J., Morales, T., Altabella, C.: Effect of salinity on soluble protein, free amino acids and nicotine contents in Nicotiana L.-Plant Soil 102: 55–60, 1987.
Dever, J.E., Jr., Bandurski, R.S., Kivilaan, A.: Partial chemical characterization of corn root cell walls.-Plant Physiol. 43: 50–56, 1968.
Enyedi, A., Yalpini, N., Silverman, P., Raskin, I.: Localization, conjugation and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus.-Proc. nat. Acad. Sci. USA 89: 2480–2484, 1992.
Fales, F.W.: The assimilation and degradation of carbohydrates by yeast cells.-J. biol. Chem. 193: 113–118, 1951.
Galbraith, D.W., Shields, B.A.: Analysis of the initial stages of plant protoplast development using 33258 Hoechst: reactivation of the cell cycle.-Physiol. Plant. 51: 380–386, 1981.
Garcia-Reina, G., Moreno, V., Luque, A.: Selection for NaCl tolerance in cell culture of three Canary Island tomato land races. I. Recovery of tolerant plantlets from NaCl-tolerant cell strains.-J. Plant Physiol. 133: 1–6, 1988.
Gordon, A.I., Ryle, G.J.A., Mitchell, D.F., Lowry, K.H. Powell, C.E.: The effect of defoliation on carbohydrate, protein and leghaemoglobin content of white clover nodules.-Ann. Bot. 58: 141–154, 1986.
Graifenberg, A., Giustiniani, O., Temperini, L., Di Paola, M.: Allocation of Na, Cl and Ca within plant tissues in globe artichoke (Cynara scolimus L.) under saline-sodic conditions.-Scientia Hort. 63: 1–10, 1995.
Greenway, H.: Effects of slowly permeating osmotic on metabolism of vacuolated and nonvacuolated tissues.-Plant Physiol. 46: 254–258, 1970.
Hamada, A.M.: Effect of exogenously added ascorbic acid, thiamin or aspirin on photosynthesis and some related activities of drought-stressed wheat plants.-In: Garab, G. (ed.): Photosynthesis: Mechanisms and Effects. Pp. 2581–2584. Kluwer Academic Publishers, Dordrecht 1998.
Hanson, A.D., Nelsen, C.E., Pedersen, A.R., Everson, E.H.: Capacity for proline accumulation during water stress in barley and its implications for breeding for drought resistance.-Crop Sci. 19: 489–493, 1979.
Hassan-Porath, E., Poljakoff-Mayber, A.: The effect of salinity on glucose absorption and incorporation by pea roots.-Plant Cell Physiol. 14: 361–368, 1973.
He, T., Cramer, G.: Growth and mineral nutrition of six rapid cycling Brassica species in response to seawater salinity.-Plant Soil 139: 285–294, 1992.
Imamul-Huq, S.M., Larher, F.: Effect of NaCl salinity on the growth and nitrogen status of nodulated cowpea (Vigna sinesis L.) and mung bean Phaseolus aureus L.-Z. Pflanzenphysiol. 112: 79–87, 1983.
Iraki, N., Carpita, N.: Extracellular polysaccharides of Nicotiana tabacum cell cultures in relation to adaptation to drought and saline stress.-Plant Physiol. 80: 500–511, 1986.
Iraki, N.M., Singh, N., Bressan, R.A., Carpita, N.C.: Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress.-Plant Physiol. 91: 39–47, 1989.
Janda, T., Szalai, G., Tari, I., Páldi, E.: Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants.-Planta 208: 175–180, 1999.
Jeschke, W.D., Wolf, O.: Effect of NaCl salinity on growth, development, ion distribution and ion translocation in castor bean (Ricinus communis L.).-J. Plant Physiol. 132: 45–53, 1988.
Kefeli, V.I.: [Vitamins and some other representatives of nonhormonal plant growth regulators.]-Prikl. Biokhim. Mikrobiol. 17: 5–15, 1981. [In Russ.]
Kennedy, B.F., De Filippis, L.F.: Physiological and oxidative response to NaCl of the salt tolerant Grevillea ilicifolia and the salt sensitive Grevillea arenaria.-J. Plant Physiol. 155: 746–754, 1999.
Kinraide, T.B.: Interactions among Ca2+, Na+ and K+ in salinity toxicity: quantitative resolution of multiple toxic and ameliorative effects.-J. exp. Bot. 50: 1495–1505, 1999.
Lopez, F., Vansuyt, G., Fourcroy, P., Cass-Delbart, F.: Accumulation of a 22 kDa protein and its mRNA in the leaves of Raphanus sativus in response to salt stress or water deficit.-Physiol. Plant. 91: 605–614, 1994.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the Folin phenol reagent.-J. biol. Chem. 193: 265–275, 1951.
Matsumoto, H., Chung, G.C.: Increase in proton-transport activity of tonoplast vesicles as an adaptive response of barley roots to NaCl stress.-Plant Cell Physiol. 29: 1133–1140, 1988.
McCree, K.J.: Whole-plant carbon balance during osmotic adjustment to drought and salinity stress.-Aust. J. Plant Physiol. 13: 33–43, 1986.
Mishra, A., Choudhuri, M.A.: Effect of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice.-Biol. Plant. 42: 409–415, 1999.
Moore, S., Stein, W.W.: Photometric ninhydrin method for use in the chromatography of amino acids.-J. biol. Chem. 176: 367–388, 1948.
Munns, R., Brady, C.I., Barlow, E.W.R.: Solutes accumulation in the apex and leaves of wheat during water stress.-Aust. J. Plant Physiol. 6: 379–389, 1979.
Neubauer, C., Yamamota, H.Y.: Mehler-peroxidase reaction mediated zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts.-Plant Physiol. 99: 1354–1361, 1992.
Perez-Alfocea, F., Guerrier, G., Estan, M.T., Bolarin, M.C.: Comparative salt responses at cell and whole-plant levels of cultivated and wild tomato species and their hybrid.-J. hort. Sci. 69: 639–644, 1994.
Perez-Alfocea, F., Bolarin, M.C., Guerrier, G.: Sucrose metabolism in NaCl-treated calli from Lycopersicon esculentum, L. pennellii and their interspecific hyprid.-J. Plant Physiol. 145: 161–167, 1995.
Santos, C., Caldeira, G.: Comperative responses of Helianthus annuus plant and calli exposed to NaCl: 1. Growth rate and osmotic regulation in intact plants and calli.-J. Plant Physiol. 155: 769–777, 1999.
Schwarzenbach, G., Biedermann, W.: Complexons, X. Alkaline earth complexes of 0,0-dihydroxyazo dyes.-Helv. chim. Acta 31: 678–687, 1948.
Serrano, R., Gaxiola, R.: Microbial models and salt stress tolerance in plants.-Crit. Rev. Plant Sci. 13: 121–138, 1994.
Skriver, K., Mundy, J.: Gene expression in response to abscisic acid and osmotic stress.-Plant Cell 2: 503–512, 1990.
Solomon, M., Ariel, R., Hodson, M.J., Mayer, A.M., Poljakoff-Mayber, A.: Ion absorption and allocation of carbon resources in excised pea roots grown in liquid medium in absence or presence of NaCl.-Ann. Bot. 59: 387–398, 1987.
Stewart, G.R., Larher, F.: Accumulation of amino acids and related compounds in relation to environmental stress.-In: Miflin B.J. (ed.): The Biochemistry of Plants. Vol. 5. Pp. 609–635. Academic Press, New York 1980.
Taiz, L.: Plant cell expansion: regulation of cell wall mechanical properties.-Annu. Rev. Plant Physiol. 35: 585–657, 1984.
Thakur, S., Rai, V.K.: Exogenously supplied amino acids and water deficits in Zea mays cultivars.-Biol. Plant. 27: 458–461, 1985.
Torres-Schumann, S., Godoy, J.A., Pentor-Toro, J.A., Moreno, F.J., Rodrigo, R.M., Garcia-Herdugo, G.: NaCl effects on tomato seed germination, cell activity and ion allocation.-J. Plant Physiol. 135: 228–232, 1989.
Van Volkenburgh, E., Boyer, J.S.: Inhibitory effects of water deficit on maize leaf elongation.-Plant Physiol. 77: 190–194, 1985.
Wakabayashi, K., Hoson, T., Kamisaka, S.: Osmotic stress suppresses cell wall stiffening and the increase in cell wall-bound ferulic and diferulic acids in wheat coleoptiles.-Plant Physiol. 113: 967–973, 1997.
Watad, A.E., Reuveni, M., Bressan, R.A., Hasegawa, P.M.: Enhanced net K+ uptake capacity of NaCl adapted cells.-Plant Physiol. 95: 1265–1269, 1991.
Williams, V., Twin, S.: Flame photometric method for sodium potassium and calcium.-In: Paech, K, Tracey, M.V (ed.): Modern Methods of Plant Analysis. Vol. V. Pp. 3–5. Springer-Verlag, Berlin 1960.
Yadav, N., Gupta, V., Yadav, V.K.: Role of benzyladenine and gibberellic acid in alleviating water-stress effect in gram (Cicer arietinum).-Indian J. agr. Sci. 67: 381–387, 1997.
Yalpini, N., Raskin: Salicylic acid: a systematic signal in induced plant decrease resistance.-Trends Microbiol. 1: 88–92, 1993.
Zhang, H., Läuchli, A.: Incorporation of [14C]glucose into cell wall polysaccharides of cotton roots: Effects of NaCl and CaCl2.-Plant Physiol. 88: 511–514, 1988.
Ziska, L.H.; Seemann, J.R., Delong, T.M.: Salinity induced limitations on photosynthesis in Prunus salicina, a deciduous tree species.-Plant Physiol. 93: 864–870, 1990.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Al-Hakimi, A., Hamada, A. Counteraction of Salinity Stress on Wheat Plants by Grain Soaking in Ascorbic Acid, Thiamin or Sodium Salicylate. Biologia Plantarum 44, 253–261 (2001). https://doi.org/10.1023/A:1010255526903
Issue Date:
DOI: https://doi.org/10.1023/A:1010255526903