Abstract
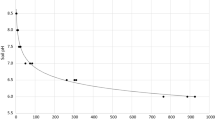
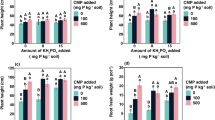
The chemical barrier to root development existing in the subsoils of acid soils is a subject of increasing interest. In order to better understand the factors involved in the amelioration of subsoil acidity, the effects of calcium sulphate, phosphogypsum and calcium carbonate on the properties of the solid and liquid phases of subsoil samples and on the growth and nutrient uptake by maize (Zea mays L.) were evaluated. The soils used were two alic red-yellow latosols, two acric dusky red latosols and one alic dark-red latosol from the State of São Paulo, Brazil. A vertical split-root technique was used in a greenhouse experiment, with the plants initially grown in a small pot with 130 g fertile soil, which was introduced in a larger pot containing 2 dm3 of the subsoil samples. The treatments consisted of a control (C) and applications of calcium carbonate (CC), calcium sulphate (CS) and phosphogypsum (PG) at the rate of 10 mmolc Ca2+ dm-3. CS and PG reduced soil acidity, but in a much smaller proportion than CC. Calcium carbonate reduced the activity of Al3+ because of the increase in pH. Total aluminum and calcium contents in the soil solution were much higher for the red-yellow latosols than for the other soils, indicating lower sorption of Ca2+ and\(SO_4^{2 - } \) in these soils. The activity of Al in the soil solution was decreased in different ways for the five soils, depending on the ionic strength and the formation of the ionic pair\(AlSO_4^ + \) and, in the case of PG, the formation of complexes of Al with F (AlF2+,\(AlF_2^ + \) and\(AlF_3^\bigcirc \)). The subsoil samples presented severe restrictions for maize root growth and all three treatments were equally effective in increasing root development, which could be attributed to the supply of calcium in one of the acric dusky red latosols and a combined effect of the amendment in reducing the activity of Al and increasing the activity of Ca in the soil solution in the other soils. As a consequence the three treatments increased in the same manner water, N and K uptake from the subsoil and the dry matter production of maize. It can be concluded that, for the soils considered in this research, phosphogypsum is an effective amendment for acid subsoils containing low calcium or toxic aluminum contents.
Similar content being viewed by others
References
Adams F and Lund Z 1966 Effect of chemical activity of soil solution aluminium on cotton root penetration of acid subsoils. Soil Sci. 101, 193-198.
Adriano D C and Donner H E 1982 Bromine, chlorine, and fluorine.InMethods of Soil Analysis. Agronomy 9. Eds. A L Page, R H Miller and D R Keeney. pp 449-483. ASA, Madison, WI.
Alcordo I S and Rechcigl J E 1993 Phosphogypsum in agriculture: A review. Adv. Agron. 49, 55-119.
Alva A K and Sumner M E 1990 Amelioration of acid soil infertility by phosphogypsum. Plant Soil 128, 127-34.
Alva A K, Sumner M E and Miller W P 1990 Reactions of gypsum or phosphogypsum in highly weathered acid subsoils. Soil Sci. Soc. Am. J. 54, 993-998.
Alva A K, Sumner M E and Miller W P 1991 Salt absorption in gypsum amended acid soils.InPlant-Soil Interactions at low pH. Eds. R J Wright, V C Baligar and R P Murrman. pp 93-97. Kluwer Academic Publishers, Dordrecht.
Alva A K, Sumner M E and Noble A D 1988 Alleviation of aluminum toxicity by phosphogypsum. Commun. Soil Sci Plant Anal. 19, 385-403.
Alva A K, Edwards D G, Asher C J and Blamey F P 1986 An evaluation of aluminum indices to predict aluminum toxicity to plant growth in nutrient solutions. Commun. Soil Sci. Plant Anal. 17, 1271-1280.
Black C A and Cameron K C 1984 Effect of leaching on soil properties and lucerne growth following lime and gypsum amendments to a soil with an acid subsoil. N.Z. J. Agric. Res. 27, 195-200.
Bremner J M and Keeney DA 1966 Determination and isotope-ratio analysis of different forms of nitrogen in soils. 3 Exchangeable ammonium, nitrate and nitrite by extraction-destillation methods. Soil Sci. Soc. Am. Proc. 30, 577-582.
Cameron R S, Ritchie G S P and Robson A D 1986 Relative toxicities of inorganic aluminum complexes to barley. Soil Sci. Soc. Am. J. 50, 1231-1236.
Freitas B J 1992 Disposal of phosphogypsum and its environmental impact.InSeminário sobre o uso de gesso na agricultura 2. pp 325-339. IBRAFOS, Uberaba, Minas Gerais (In Portuguese).
Gonzalez-Erico E, Kamprath E J, Naderman G C and Soares W V 1979 Effect of depth of lime incorporation on the growth of corn on an Oxisol of Central Brazil. Soil Sci. Soc. Am. J. 43, 1155-1158.
Hue N V, Adams F and Evans C E 1985 Sulfate retention by an acid BE horizon of an Ultisol. Soil Sci Soc. Am. J. 49, 1196-1200.
Ismail H, Shamshuddin N and Syed Omar S R 1993 Alleviation of soil acidity in Ultisol and Oxisol for corn growth. Plant Soil 151, 55-65.
Marcano-Martinez E and McBride M B 1989 Calcium and sulfate retention by two oxisols of the Brazilian cerrado. Soil Sci. Soc. Am. J. 53, 63-69.
McLay C D A and Ritchie G S P 1993 Effect of gypsum on wheat growth in pots containing an acidic subsoil.InPlant Nutrition: From Genetic Engineering to Field Practice. pp 747-750. Kluwer Academic Publishers, Dordrecht.
Moore C S and Ritchie G S P 1988 Aluminium speciation and pH of an acid soil in the presence of fluoride. J. Soil Sci. 39, 1-8.
Olmos J L J and Camargo M N 1979 Occurrence of toxic aluminum in the soils of Brazil, characterization and distribution. Ciênc. Cult. 28, 171-180 (In Portuguese).
Pavan M A and Bingham F T 1982 Toxicity of aluminium to coffee seedlings grown in nutrient solution. Soil Sci. Soc. Am. J. 46, 993-997.
Pavan M A, Bingham F T and Pratt P F 1982 Toxicity of aluminium to coffee in Ultisols and Oxisols amended with CaCO3, MgCO3 and CaSO4. 2H2O. Soil Sci. Soc. Am. J. 46, 1201-1207.
Quaggio J A, van Raij B and Malavolta E 1985 Alternative use of the SMP-buffer solution to determine lime requirement of soils. Commun. Soil Sci. Plant Anal. 16, 245-260.
Raij B van and Peech M 1972 Eletrochemical properties of some oxisols and alfisols of the tropics. Soil Sci. Soc. Am. J. 36, 587- 597.
Raij B van and Valadares J M A S 1974 Analysis of major elements in rocks, clays and soils. Instituto Agronomico Campinas, São Paulo. 23 p. (In Portuguese).
Raij B van, Cantarella H and Furlani P R 1988 Influence on soil reaction of ammonium and nitrate uptake by sorghum plants, in presence or absence of gypsum. R. Bras. Ci. Solo 12, 131-136 (In Portuguese).
Raij B van, Quaggio J A, Cantarella H, Ferreira ME, Lopes A S and Bataglia O C 1987 Chemical analysis for soil fertility. Funda¸cão Cargill, Campinas, Sao Paulo. 170 p. (In Portuguese).
Reeve N G and Sumner M E 1972 Amelioration of subsoil acidity in Natal Oxisols by leaching surface applied amendments. Agrochemophysica 4, 1-6.
Rhoades J D 1982 Soluble salts.InMethods of Soil Analysis. Agronomy 9. Eds. A L Page, R H Miller and D R Keeney. pp 167-179. ASA, Madison, WI.
Ritchey K D, Silva J E and Costa U F 1982 Calcium deficiency in clayey B horizonts of savannah Oxisols. Soil Sci. 133, 378-382.
Ritchey K D, Souza D H G, Lobato E and Correa O 1980 Calcium leaching to increase rooting depth in a Brazilian Savannah Oxisol. Agron. J. 72, 40-44.
Shainberg J, Summer M E, Miller W P, Farina M P W, Pavan M A and Fey M V 1989 Use of gypsum on soils: a review. Adv. Soil Sci. 9, 1-111.
Sposito G and Mattigod S V 1980 Geochem: a computer program for the calculation of chemical equilibria in soil solutions and other natural water systems. University of California, Kearney Foundation of Soil Science, Riverside. 82 p.
Sumner M E 1993 Gypsum and acid soils: The world scene. Adv. Agron. 51, 1-32.
Sumner ME 1994 Measurement of soil pH: problems and solutions. Commun. Soil Sci. Plant Anal. 25, 859-879.
Sumner M E, Shahandeh H, Bouton J and Hammel J 1986 Amelioration on acid soil profile through deep liming and surface application of gypsum. Soil Sci. Soc. Am. J. 50, 1254-1278.
Tabatabai M A and Bremner J M 1970 Asimple turbidimetric method of determining total sulfur in plant materials. Agron. J. 62, 805- 806.
Tennant D 1975 A test of a modified line intersect method of estimating root lenght. J. Ecol. 63, 995-1001.
Wright R J, Hern J L, Baligar V C and Bennet O L 1985 The effects of surface applied amendments on barley root growth in an acid subsoil. Commun. Soil Sci. Plant Anal. 16, 179-192.
Rights and permissions
About this article
Cite this article
Carvalho, M., van Raij, B. Calcium sulphate, phosphogypsum and calcium carbonate in the amelioration of acid subsoils for root growth. Plant and Soil 192, 37–48 (1997). https://doi.org/10.1023/A:1004285113189
Issue Date:
DOI: https://doi.org/10.1023/A:1004285113189