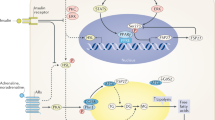
Abstract
GH and PRL are both implicated in adipose tissue development. Whilst direct effects of GH have been clearly demonstrated, direct effects of PRL have been subject to considerable debate. Recent studies have however provided compelling evidence for PRL receptors on adipocytes and in vitro effects on leptin and lipoprotein lipase activity have been demonstrated. Quantitatively however these effects of PRL are less significant than those of GH and the most pronounced effects of PRL on adipose tissue are indirect, for example, during lactation, when prolactin drives milk synthesis which results in a homeorhetic shift towards lipid mobilization from adipose tissue to support milk production. GH also exhibits such homeorhetic effects, most notably in ruminants, but also clearly has direct, insulin-antagonistic, metabolic effects. The roles of GH and PRL on adipocyte proliferation and differentiation have also been controversial, with GH stimulating adipocyte differentiation in vitro in cell lines whilst stimulating proliferation and inhibiting differentiation of primary cell cultures. Examination of adipose tissue development in PRLRko and GHRko mice has revealed roles for both hormones. PRLRko mice show impaired development of both internal and subcutaneous adipose tissue due to decreased numbers of adipocytes. In contrast, GHRko mice exhibit major decreases in the number of internal (parametrial) adipocytes whereas subcutaneous adipocytes develop almost normally. This leads to major changes in the sites of adipose tissue accretion and bears interesting parallels with the adipose tissue redistribution which occurs in HIV-induced lipodystrophy. Such individuals exhibit a central obesity which can be partially corrected by GH treatment. However, recent studies suggest that this may be a physiological response in which adipose tissue sites containing lymphoid tissue (such as mesenteric) show preservation of adipose tissue perhaps to support augmented immune responses.
Similar content being viewed by others
References
Freemark M, Fleenor D, Driscoll P, Binart N, Kelly PA. Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinol 2001;142:532-537.
Do Nascimento O, Illic V, Williamson DH. Re-examnation of the putative roles of insulin and prolactin in the regulation of lipid deposition and lipogenesis in vivo in mammary gland and white and brown adipose tissue of lactating rats and litterremoved rats. Biochem J 1989;,258:273-278..
Bandyopadhyay GK, Lee LY, Guzman RC, Nandi A. Effects of reproductive status on lipid mobilization and linoleic acid metabolism in mammary glands. Lipids 1995;30:155-162.
Turtle JR, Kipnis DM. The lipolytic action of human placental lactogen on isolated fat cells. Biochim Biophys Acta 1967;144:583-593.
Genazzani AR, Benuzzi-Badoni M, Felber JP. Human chorionic somatomammotropin: Lipolytic action of a pure preparation on isolated fat cells. Metab Clin Exp 1969;18:593-598.
Strange RC, Swyer GI. The effect of human placental lactogen on adipose tissue lipolysis. JEndocrinol 1974;61:147-152.
Williams C, Coltart TM. Adipose tissue metabolism in pregnancy: The lipolytic effect of human placental lactogen. Br J Obstet Gynecol 1978;85:43-46.
Iliou JP, Damarne Y. Evolution of the sensitivity of isolated adipocytes of ewes to the lipolytic effects of different stimuli during pregnancy and lactation. Int J Biochem 1987;19:253-258.
Houseknecht KL, Bauman DE, Vernon RG, Byatt JC, Collier RJ. Insulin-like growth factors 1 and 2, somatotropin, prolactin and placental lactogen are not acute effectors of lipolysis in ruminants. Dom Anim Endocrinol 1996;13:2239-249.
Fielder PJ, Talamantes F. The lipolytic effects of mouse placental lactogen I, mouse prolactin, and mouse growth hormone on adipose tissue from virgin and pregnant mice. Endocrinol 1987;121:493-497.
Gerardo-Gettens T, Moore BJ, Stern JS, Horwitz BA. Prolactin stimulates food intake in a dose-dependent manner. Am J Physiol 1989;256:R276-R280.
Byatt JC, Staten NR, Sasgiver WJ, Kostelc JG, Collier RJ. Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Am J Physiol 1993;264:E986-E992.
Sauve D, Woodside B. The effect of central administration of prolactin on food intake in virgin female rats is dosedependent, occurs in the absence of ovarian hormones and the latency to onset varies with feeding regimen. Brain Res 1996;729:75-81.
Buntin JD, Hnasko RM, Zuzick PH. Role of the ventromedial hypothalamus in prolactin-induced hyperphagia in ring doves. Physiol Behav 1999;66:255-261.
Jarrett JC, Ballejo G, Saleem TH, Tsibris JCM, Spellacy WN. The effect of prolactin and relaxin on insulin binding by adipocytes from pregnant women. Am J Obstet Gynecol 1984;149:250-255.
Ryan EA, Enns L Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab 1988;67:341-347.
Hamosh M, Clary TR, Chernick SS, Scow RO. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta 1970;210:473-482.
Zinder O, Hamosh M, Fleck TRC, Scow RO. Effect of prolactin on lipoprotein lipase in mammary gland and adipose tissue of rats. Am J Physiol 1974;226:774-778.
Agius L, Robinson AM, Girard JR, Williamson DH. Alterations in the rate of lipogenesis in vivo in maternal liver and adipose tissue on premature weaning of lactating rats: A possible regulatory role of prolactin. Biochem J 1979; 180:689-692.
Flint DJ, Clegg RA, Vernon RG. Prolactin and the regulation of adipose tissue metabolism during lactation in rats. Molt Cell Endocrinol 1981;22:265-275.
Jensen DR, Gavigan S, Sawicki V, Witsell DL, Eckel RH, Nevile MC. Regulation oflipoprotein lipase activity and mRNA in the mammary gland of the lactating mouse. Biochem J 1994;298:321-327.
Matsuda M, Mori T, Sassa S, Sakamoto S, Kyun Park M, Kawashima S. Chronic effect of hyperprolactinemia on blood glucose and lipid levels in mice. Life Sci 1996;58:1171-1177.
Stanton JM. Weight gain associated with neuroleptic medication. A review. Schizophr Bull 1995;21:463-472.
Baptista T. Body weight gain induced by antipsychotic drugs: Mechanisms and management. Acta Psychiatr Scand 1999;100:3-16.
Greenman Y, Tordjman K, Stern N. Increased body weight associated with prolactin secreting pituitary adenomas: Weight loss with normalization of prolactin levels. Clin Endocrinol 1998;48:547-553.
Ferriera MF, Sobrinho LG, Santos MA, Sousa MF, Uvnas-Moberg K. Rapid weight gain, at least in some women, is an expression of a neuroendocrine state characterized by reduced hypothalamic dopaminergic tone. Psychoneuroendocrinology 1998;23:1005-1013.
Baptista T, Araujo de Baptista E, Altemus M, Weiss SRB, Hernandez L. Tamoxifen prevents sulpiride-induced obesity in rats. Pharmacol Biochem Behav 1997a;57:215-222.
Baptista T, López MA, Teneud L, Contreras Q, Araujo E, Weiss S and others. Amantadine in the treatment of Prolactin, GH and Adipose Tissue Development 101 neuroleptic-induced obesity in rats: Behavioural, endocrn and neurochemical correlates. Pharmacopsychiatry 1997b;30:43-54.
Baptista T, Hernández L, Parada MA. Long term administration of some antipsychotic drugs increases body weight and feeding in rats. Are Dopamine D2 receptors involved? Pharmacol Biochem Behav 1987;27:399-405.
Petty RG. Prolactin and antipsychotic medication: Mechanisms of action. Schizophr Res 1999;35:S67-S73.
Kapur S, Remington G. Atypical antipsychotics: New directions and new challenges in the treatment of schizophrenia. Annual Reviews of Medicine 2001;52:503-517.
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly P. Prolactin (PRL) and its receptor. Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrinol Rev 1998;19:225-268.
Ohkubo T, Tanaka M, Nakashima K, Talbot RT, Sharp PJ. Prolactin receptor gene expression in the brain and peripheral tissues in broody and nonbroody breeds of domestic hen. Gen Comp Endocrinol 1998;109:60-68.
Clarke DL, Linzer DIH. Changes in prolactin receptor expression during pregnancy in the mouse ovary. Endocrinol 1993;133:224-232.
Das R, Vonderhaar BK. Transduction of prolactin's (PRL) growth signal through both long and short forms of the PRL receptor. Mol Endocrinol 1995;9:1750-1759.
Lebrun JJ, Ali S, Goffin V, Ullrich A, Kelly PA. A single phosphotyrosine residue of the prolactin receptor is responsible for activation of gene transcription. Proc NaUl Acad Sci (USA) 1995;92:4031-4035.
Lebrun JJ, All S, Ullrich A, Kelly PA. Proline-rich sequencemediated JAK2 association to the prolactin receptor is required but not sufficient for signal transduction. J Biol Chem 1995;270:10664-10670.
Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of T'Ir694 of STATS (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J 1994;13:4361-4369.
Ling C, Hellgren G, Gevre-Medhin M, Dillner K, Wennbo H, Carlsso B, Billig H. Prolactin (PRL) receptor gene expression in mouse adipose tissue: Increases during lactation and in PRL-transgenic mice. Endocrinology 2000; 141(10):3564-3572.
Ling C, Billig H. PRL Receptor-mediated effects in female mouse adipocytes: PRL induces suppressors of cytokine signaling expression and suppresses insulin-induced leptin production in adipocytes in vitro. Endocrinology 2001;142(11):4880-4890.
Ling C, Svensson L, Oden B, Weijdegard B, Eden B, Eden S, Billig H. Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J Clin Endocrinol Metab 2003;88(4):1804-1808.
Etherton TD, Bauman DE. Biology of somatotropin in growth and lactation of domestic animals. Physiol Rev 1998;78:745.
Walton PE, Etherton TD. Stimulation of lipogenesis by insulin in swine adipose tissue: Antagonism by porcine growth hormone. J Anim Sci 1986;62:1584.
Walton PE, Etherton TD, Chung CS. Exogenous pituitary and recombinant growth hormones induce insulin and insulin-like growth factor 1 resistance in pig adipose tissue. Domest Anim Endocrinol 1987;4:183.
Walton PE, Etherton TD, Evock CM. Antagonism of insulin action in culturedpig adipose tissue by pituitary and recombinant porcine growth hormone: Potentiation by hydrocortisone. Endocrinol 1986;118:2577.
Dunshea FR, Harris DM, Bauman DE, Boyd RD, Bell AW. Effect of porcine somatotropin on in vivo glucose kinetics and lipogenesis in growing pigs. J Anim Sci 1992;70:141.
Etherton TD, Louveau I, Sorensen MT, Chaudhuri S. Mechanisms by which somatotropin decreases adipose tissue growth. Am J Clin Nutr 1993;58(Suppl):287S.
Etherton TD, Smith SB. Somatotropin and ß-adrenergic agonists: Their efficacy and mechanisms of action. J Anim Sci 1991;69(Suppl 2):2.
Wray-Cahen D, Bell AW, Boyd RD et al. Nutrient uptake by the hindlimb of growing pigs treated with procine somatotropin and insulin. J Nutr 1995;25:125.
Magri KA, Adamo M, Leroith D, Etherton TD. The inhibition of insulin action and glucose metabolism by porcine growth in porcine adipocytes is not the result of any decrease in insulin binding or insulin receptor kinase activity. Biochem J 1990;266:107.
Li C, Simpson ME, Evans HM. Influence of growth and adrenocorticotropic hormones on the body composition of hypohysectomized rats. Endocrinol 1949;44:71-75.
Scow R. Effect of growth hormone and thyroxine on growth and chemical composition of muscle, bone and other tissues in thryoidectomized-hypophysectomized rats. Am J Physiol 1959;196:859-865.
Oberbauer AM, Stern JS, Johnson PR, Horwitz BA, German JB, Phinney SD, Beermann DH, Pomp D, Murray JD. Body composition of inactivated growth hormone (oMtla-oGH) transgenic mice: Generation of an obese phenotype. Growth Dev Aging 1997;61:169-179.
Wabitsch M, Hauner H, Heinze E, Teller WM. The role of growth hormone/inmsulin-like growth factors in adipocyte differentiation. Metabolism 1995;44:45-49.
Bonnet FP, Vanderscheuren-Lodeweyckx M, Eeckels RT, Malvaux P. Subcutaneous adipose tissue and lipids in blood in growth hormone deficiency before and after treatment with growth hormone. Pediatr Res 1974;8:800-805.
Salomon F, Cuneo RC, Hesp R, Sonksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 1989,321:1797-1803.
Rosen T, Bosaeus I, Tolli J, Lindstedt G, Bengtsson BA. Increased body fat mass and decreased extracellular fluid volume in adults with growth hormone deficiency. Clin Endocrinol (Oxf) 1993;38:63-71.
Zachmann M, Fernandez F, Tassinari D, Thakker, R, Prader A. Anthropometric measurements in patients with growth hormone deficiency before treatment with human growth hormone. Eur J Pediatr 1980;133:277-282.
Bengtsson BA, Eden S, Lonn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tolli J, Sjostrom L, Isaksson OG. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab 1993;76:309-317.
Snyder DK, Underwood LE, Clemmons DR. Anabolic effects of growth hormone in obese diet-restricted subjects are dose dependent. Am J Clin Nutr 1990;52:431-437.
Skaggs SR, Crist DM. Exogenous human growth hormone reduces body fat in obese women. Horm Res 1991;35:19-24.
Nam SY, Marcus C. Growth hormone and adipocyte function in obesity. Horm Res 2000;53(51):87-97.
Johannsson G, Rosen T, Lonn L, Bengtsson BA. Effects of recombinant human growth hormone on adipose tissue in adults with growth hormone deficiency. Acta Paediatr Suppl December 1994;406:60-63.
Richelsen B. Action of growth hormone in adipose tissue. Horm Res 1997;48:105-110.
Jorgensen JO, Vahl N, Fisker S, Norrelund H, Nielsen S, Dall R, Christiansen JS. Somatopause and adiposity. Horm Res 1997;48:101-104.
Shadid S, Jensen MD. Effects of growth hormone administration in human obesity. Obes Res 2003;11(2):170-175.
Fagin KD, Lackey SL, Reagan CR, DiGirolama M. Specific binding of GH by rat adipocytes. Endocrinol 1980;107:608-615.
Vilkman K, Carlsson B, Billig H, Eden S. Expression and regulation of growth hormone (GH) receptor messenger ribonucleic acid (mRNA) in rat adipose tissue, adipocytes and adipocyte precursor cells: GH regulation of GH receptor mRNA. Endocrinol 1991;129:1155-1161.
Mertani HC, Delehaye-Zervas Martini JF, Postel-Vinay MC, Morel G. Localization of growth hormone receptor messenger RNA in human tissues. Endocrine 1995;3:135-142.
Green H, Morikawa M, Nixon T. A dual effector theory of growth-hormone action. Differentiation 1985;29:195-198.
Morikawa M, Nixon T, Green H. Growth hormone and the adipose conversion of 3T3 cells. Cell 1982;29:783-789.
Wabitsch M, Heinze E, Hauner H, Shymko RM, Teller WM, De Meyrs P, Hondo MM. Biological effects of human growth hormone in rat adipocyte precursor cells and newly differentiated adipocytes in primary culture. Metabolism 1996a;45:34-42.
Wabitsch M, Braun S, Hauner H, Heinze E, Iondo MM, Shymko R, De Meyrs P, Teller WM. Mitogenic and antiadipogenic properties of human growth hormone in differentiating human adipocyte precursor cells in primary culture. Pediatr Res 1996b;40:450-456.
Kassem M, Blum W, Ristelli J, Mosekilde L, Eriksen EF. Growth hormone stimulates proliferation and differentiation of normal human osteoblast-like cells in vitro. Calcif Tissue Int Mar 1993;52:222-226.
Nishiyama K, Sugimoto T, Kaji H, Kanatani M, Kobayashi T, Chihara K. Stimulatory effect of growth hormone on bone resorption and osteoclast differentiation. Endocrinol 1996;137:35-41.
Ewton DZ, Florini JR. Relative effects of the somatomedins, multiplication-stimulating activity, and growth hormone on myoblasts and myotubes in culture. Endocrinol 1980;106:577-583.
Corin RE, Guller S, Wu KY, Sonenberg M. Growth hormone and adipose differentiation: Growth hormone-induced antimitogenic state in 3T3-F442A preadipose cells. Proc Natl Acad Sci (USA) 1990;87:7507-7511.
Tang B, Jeoung DI, Sonenberg M. Effect of human growth hormone and insulin on [3H] thymidine incorporation, cell cycle progression, and cyclin D expression in 3T3-F442A preadipose cells. Endocrinol 1995;136:3062-3069.
Kim So, Houtman JC, Jiang J, Ruppert JM, Bertics PJ, Frank SJ. Growth hormone-induced alteration in ErbB-2 phosphorylation status in 3T3-F442A fibroblasts. J Biol Chem 1999;274:36015-36024.
Wiepz GJ, Houtman JC, Cha D, Bertics PJ. Growth hormone attenuation of epidermal growth factor-induced mitogenesis. J Cell Physiol 1999;173:44-53.
Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Ed M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Der 1997;11(2):167-178.
Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Oke S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by target disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci 1997; 94:13215-13220.
Flint DJ, Gardner MJ. Influence of growth hormone deficiency on growth and body composition in rats: Site-specific effects upon adipose tissue development. J Endocrinol 1993;137(2):203-211.
LaFranchi S, Hanna CE, Torresani T, Schoenle E, Illig R. Comparison of growth hormone binding and metabolic response in rat adipocytes of epididymal, subcutaneous, and retroperitoneal origin. Acta Endocrinol (Copenh) 1985;110:50-55.
Landron D, Gugail I, Ardouin B, Quignard-Boulange A, Postel-Vinay MC. Growth hormone binding to cultured preadipocytes from obese fa/fa rats increases during cell differentiation. Horm Metab Res 1987;19:403-406.
Zou L, Menon RK, Sperling MA. Induction of mRNAs for the growth hormone receptor gene during mouse 3t3-L1 preadipocyte differentiation. Metabolism 1997;46:114-118.
Pond CM. Physiological specialisation of adipose tissue. Prog Lipid Res 1999;38(3):225-248.
Pond CM, Mattacks CA. The activation of the adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine 2002;17(3):131-139.
Mattacks CA, Sadler D, Pond CM. The cellular structure and lipid/protein composition of adipose tissue surrounding chronically stimulated lymph nodes in rats. J Anat 2003;202(6):551-561.
Pond CM. Paracrine relationships between adipose and lymphoid tissue implications for the mechanism of HIV-associated adipose redistribution syndrome. Trends Immunol 2003;24(1):13-18.
Rennie MJ. Claims for the anabolic effects of growth hormone: A case of the Emperor's new clothes? Br J Sports Med 2003;37:100-105.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Flint, D.J., Binart, N., Kopchick, J. et al. Effects of Growth Hormone and Prolactin on Adipose Tissue Development and Function. Pituitary 6, 97–102 (2003). https://doi.org/10.1023/B:PITU.0000004800.57449.67
Issue Date:
DOI: https://doi.org/10.1023/B:PITU.0000004800.57449.67