Abstract
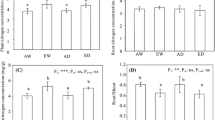
The carbon/nutrient balance hypothesis has recently been interpreted to predict that plants grown under elevated CO2 environments will allocate excess carbon to defense, resulting in an increase in carbon-based secondary compounds. A related prediction is that, because plant growth will be increasingly nitrogen-limited under elevated CO2 environments, plants will allocate less nitrogen to defense, resulting in decreased levels of nitrogen-containing secondary compounds. We present the first evidence of decreased investment in nitrogen-containing secondary compounds for a plant grown under elevated CO2. We also present evidence that plant response is species-specific and is not correlated with changes in leaf nitrogen content or leaf carbon–nitrogen ratio. When three crucifers were grown at 724 ± 8 ppm CO2, total foliar glucosinolate content decreased significantly for mustard, but not for radish or turnip. Glucosinolate content of the second and fourth youngest mustard leaves decreased by 45% and 31%, respectively. In contrast, no significant change in total glucosinolate content was observed in turnip or radish leaves, despite significant decreases in leaf nitrogen content. Total glucosinolate content differed significantly among leaves of different age; however, the trend differed among species. For both mustard and turnip, glucosinolate content was significantly higher in older leaves, while the opposite was true for radish. No significant CO2 × leaf age interaction was observed, suggesting that intraplant patterns of allocation to defense will not change for these species. Changes in nitrogen allocation strategy are likely to be species-specific as plants experience increasing atmospheric CO2 levels. The ecological consequences of CO2-induced changes in plant defensive investment remain to be investigated.
Similar content being viewed by others
REFERENCES
AYRES, M. P. 1993. Plant defense, herbivory, and climate change, pp. 75–94. in P. M. Kareiva, J. G. Kingsolver, and R. B. Huey (eds.). Biotic Interactions and Global Change. Sinauer Associates, Sunderland, Massachusetts.
BALDWIN, I. T., and OHNMEISS, T. E. 1994. Coordination of photosynthetic and alkaloidal responses to damage in uninducible and inducible Nicotiana sylvestris. Ecology 75:1003–1014.
BAXTER, R., ASHENDEN, T. W., and FARRAR, J. F. 1994. Effects of elevated carbon dioxide on three grass species from montane pasture. II. Nutrient uptake, allocation and efficiency of use. J. Exp. Bot. 45:1267–1278.
BAZZAZ, F. A. 1990. The response of natural ecosystems to the rising global CO2 levels. Annu.Rev. Ecol. Syst. 21:167–196.
BENTLEY, B. L., and JOHNSON, N. D. 1991. Plants as food for herbivores: the roles of nitrogen fixation and carbon dioxide enrichment, pp. 257–272, in P. W. Price, T. M. Lewinsohn, G. W. Fernandas, and W. W. Benson (eds.). Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley & Sons, New York.
BERNAYS, E. A. (ed.). 1992. Insect-Plant Interactions, Vol. IV. CRC Press, Boston.
BLAU, P. A., FEENY, P., and CONTRADO, L. 1978. Allylglucosinolate and herbivorous caterpillars: A contrast in toxicity and tolerance. Science 200:1296–1298.
BRYANT, J. P., CHAPIN, F. S., and KLEIN, D. R. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368.
CHEW, F. S. 1988a. Searching for defensive chemistry in the Cruciferae, or, do glucosinolates always control the interactions of Cruciferae with their potential herbivores and symbionts? No! pp. 81–112, in K. C. Spencer (ed.). Chemical Mediation of Coevolution. Academic Press, New York.
CHEW, F. S. 1988b. Biological effects of glucosinolates, pp. 155–181, in H. G. Cutler (ed.). Biologically Active Natural Products for Potential Use in Agriculture. American Chemical Society, Washington, D.C.
CHEW, F. S., and RENWICK, J. A. A. 1995. Chemical ecology of hostplant choice in Pieris butterflies, pp. 214–248, in R. T. Cardé, and W. J. Bell (eds.). Chemical Ecology of Insects 2. Chapman and Hall, New York.
COLEMAN, J. S., MC CONNAUGHAY, K. D. M., and BAZZAZ, F. A. 1993. Elevated CO2, and plant nitrogen-use: is reduced tissue nitrogen concentration size-dependent? Oecologia 93:195–200.
DAVID, W. A., and GARDINER, B. O. 1962. Mustard oil glycosides as feeding stimulants for Pieris brassicae larvae in a semisynthetic diet. Bull. Entomol. Res. 53:91–109.
DRAKE, B. G., LEADLEY, P. W., ARP, W. J., NASSIRY, D., and CURTIS, P. S. 1989. An open top chamber for field studies of elevated atmospheric CO2 concentration on salt marsh vegetation. Funct. Ecol. 3:363–371.
EPRON, D., LIOZON, R., and MOUSSEAU, M. 1996. Effects of elevated CO2 concentration on leaf characteristics and photosynthetic capacity of beech (Fagus sylvalica) during the growing season. Tree Physiol. 16:425–432.
ERICKSON, J. M., and FEENY, P. 1974. Sinigrin: A chemical barrier to the black swallowtail butterfly, Papilio polyxenes. Ecology 55:103–111.
FAJER, E. D., BOWERS, M. D., and BAZZAZ, F. A. 1989. The effects of enriched carbon dioxide atmospheres on plant-herbivore interactions. Science 243:1198–1200.
FAJER, E. D., BOWERS, M. D., and BAZZAZ, F. A. 1991. The effects of enriched CO2 atmospheres on the buckeye butterfly, Junonia coenia. Ecology 72:751–754.
FAJER, E. D., BOWERS, M. D., and BAZZAZ, F. A. 1992. The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. Am. Nat. 140:707–723.
FEENY, P. 1976. Plant apparency and chemical defense. Recent Adv. Phytochem. 10:1–40.
FEENY, P. 1977. Defensive ecology of the Cruciferae. Ann. Mo. Bot. Gard. 64:221–234.
FEENY, P., PAAUWE, K., and DEMONG, N. 1970. Flea beetles and mustard oils: Hostplant specificity of Phyllotreta cruciferae and P. striolata adults (Coleoptera: Chrysomelidae). Ann. Entomol Soc. Am. 63:832–841.
GATES, D. M. 1993. Climate Change and Its Biological Consequences. Sinauer Associates, Sunderland, Massachusetts.
GRAEDEL, T. E., and CRUTZEN, P. 1993. Atmospheric Change: An Earth System Perspective. W. H. Freeman, New York.
HEANEY, R. K., and FENWICK, G. R. 1981. A micro-column method for the rapid determination of total glucosinolate content of cruciferous material. Z. Pflanzenzuchtg. 87:89–95.
HOLLEY, R. A., and JONES, J. D. 1985. The role of myrosinase in the development of toxicity toward Nematospora in mustard seed. Can. J. Bot. 63:521–526.
HOUGHTON, J. T., JENKINS, G. J., and EPHRAUMS, J. J. 1990. Climate Change: The IPCC Scientific Assessment. Cambridge University Press, Cambridge, UK.
HUANG, X., RENWICK, J. A. A., and SACHDEV-GUPTA, K. 1994. Oviposition stimulants and deterrents control differential acceptance of Alliaria petiolata by Pieris rapae and P. napi oleracea. Chemoecology 5/6:79–87.
JOHNSON, R. H., and LINCOLN, D. E. 1990. Sagebrush and grasshopper responses to atmospheric carbon dioxide. Oecologia 84:103–110.
JULKUNEN-TIITTO, R., TAHVANAINEN, J., and SILVOLA, J. 1993. Increased CO2 and nutrient status changes affect phytomass and the production of plant defensive secondary chemicals in Salix myrsinifolia (Salisb.). Oecologia 95:495–498.
LARSEN, P. O. 1981. Glucosinolates, pp. 501–525, in E. E. Conn (ed.). The Biochemistry of Plants. Academic Press, New York.
LAVOLA, A., and JULKUNEN-TIITTO, R. 1994. The effect of elevated carbon dioxide and fertilization on primary and secondary metabolites in birch, Betula pendula (Roth). Oecologia 99:315–321.
LINCOLN, D. E., and D. COUVET. 1989. The effect of carbon supply on allocation to allelochemicals and caterpillar consumption of peppermint. Oecologia 78:112–114.
LINCOLN, D. E., SIONIT, N., and STRAIN, B. 1984. Growth and feeding response of Pseudoplusia includens (Lepidoptera: Noctuidae) to host plants grown in controlled carbon dioxide atmospheres. Environ. Entomol. 13:1527–1530.
LINDROTH, R. L., KINNEY, K. K., and PLATZ, C. L. 1993. Responses of deciduous trees to elevated atmospheric CO2: Productivity, phytochemistry, and insect performance. Ecology 74:763–777.
LOUDA, S., and MOLE, S. 1991. Glucosinolates: chemistry and ecology, pp. 123–164, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions With Secondary Plant Metabolites. Vol. I: The Chemical Participants. Academic Press, New York.
LOUDA, S., and RODMAN, J. 1983. Concentration of glucosinolates in relation to habitat and insect herbivory for the native crucifer, Cardamine cordifolia. Biochetn. Syst. Ecol. 11:199–207.
MA, W. C., and SCHOONHOVEN, L. M. 1973. Tarsal chemosensory hairs of the large white butterfly Pieris brassicae and their possible role in oviposition behavior. Entomol. Exp. Appl. 16:343–357.
MC CLOSKEY, C., and ISMAN, M. B. 1993. Influence of foliar glucosinolates in oilseed rape and mustard on feeding and growth of the Bertha armyworm, Mamestra configurata Walker. J.Chem. Ecol. 19:249–266.
MC CONNAUGHAY, K. D. M., BERNSTON, G. M., and BAZZAZ, F. A. 1993. Limitations to CO2-induced growth enhancement in pot studies. Oecologia 94:550–557.
MOONEY, H. A., DRAKE, B. G., LUXMOORE, R. J., OECHEL, W. C., and PITELKA, L. F. 1991. Predicting ecosystem responses to elevated CO2 concentrations. Bioscience 41:96–104.
PENUELAS, J., ESTIARTE, M., KIMBALL, B. A., IDSO, S. B., PINTER, P. J., WALL, G. W., GARCIA,R. L., HANSAKER, D. J., LAMORTE, R. L., and HENDRIX, D. L. 1996. Variety of responses of plant phenolic concentration to CO2 enrichment. J. Exp. Bot. 47:1463–1467.
PIVNICK, K. A., JARVIS, B. J., and SLATER, G. P. 1994. Identification of olfactory cues used in host-plant finding by diamondback moth, Plutella xyloslella (Lepidoptera: Plutellidae). J. Chem. Ecol. 20:1407–1427.
REED, D. W., PIVNICK, K. A., and UNDERHILL, E. W. 1989. Identification of chemical oviposition stimulants for the diamondback moth, Plutella xylostella, present in three species of Brassicaceae. Entomol. Exp. Appl. 53:277–286.
RENWICK, J. A. A. 1988. Comparative mechanisms of host selection by insects attacking pine trees and crucifers, pp. 303–316, in K. C. Spencer (ed.). Chemical Mediation of Coevolution. Academic Press, New York.
RENWICK, J. A. A. 1997. Diversity and dynamics of crucifer defenses against adults and larvae of cabbage butterflies, pp. 57–79, in J. T. Romeo, J. A. Saunders, and P. Barbosa (eds.). Phytochemical Diversity and Redundancy in Ecological Interactions. Plenum Press, New York.
RHOADES, D. F., and CATES, R. G. 1976. Toward a general theory of plant anti-herbivore chemistry. Phytochemistry 10:168–213.
RODMAN, J. E. 1981. Divergence, convergence, and parallelism in phytochemical characters: The glucosinolate-myrosinase system, pp. 43–79, in D. A. Young and D. A. Siegler (eds.). Phytochemistry and Angiosperm Phylogeny. Praeger, New York.
RODMAN, J. E., and CHEW, F. S. 1980. Phytochemical correlates of herbivory in a community of native and naturalized Cruciferae. Biochem. Syst. Ecol. 8:43–50.
ROMEO, J. T., SAUNDERS, J. A., and BARBOSA, P. (eds.). 1997. Phytochemical Diversity and Redundancy in Ecological Interactions. Plenum Press, New York.
ROSENTHAL, G. A., and BERENBAUM, M. R. (eds.). 1991. Herbivores: Their Interactions with Secondary Plant Metabolites. Vol. I: The Chemical Participants. Academic Press, New York.
ROSENTHAL, G. A., and JANZEN, D. H. (eds.). 1979. Herbivores: Their Interaction with Secondary Plant Metabolites, Academic Press, New York.
ROTHSCHILD, M. 1987. Speculations concerning the large white butterfly (Pieris brassicae L.): Do females assess the number of suitable host plants present? pp. 175–192, in R. F. Chapman, E. A. Bernays, and J. Stoffolano (eds.). Perspectives in Chemoreception and Behavior. Springer-Verlag, New York.
SCHOONHOVEN, L. M. 1972. Secondary plant substances and insects. Phytochemistry 5:197–224.
SCHOONHOVEN, L. M., and BLOM, F. 1988. Chemoreception and feeding behavior in a caterpillar: Towards a model of brain functioning in insects. Entomol. Exp. Appl. 49:123–129.
SIEMENS, D. H., and MITCHELL-OLDS, T. 1996. Glucosinolates and herbivory by specialists (Coleoptera: Chrysomelidae, Lepidoptera: Plutellidae): Consequences of concentration and induced resistance. Environ. Entomol. 25:1344–1353.
SPENCER, K. C. (ed.). 1988. Chemical Mediation of Coevolution. Academic Press, New York.
STRAIN, B. R., and CURE, J. D. (eds.). 1985. Direct Effects of Increasing Carbon Dioxide on Vegetation. US Department of Energy DOE/ER-0238, Washington, D.C.
VAN ETTEN, C. H., and H. L. TOOKEY. 1991. Chemistry and biological effects of glucosinolates, pp. 471–500, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites. Vol. I: The Chemical Participants. Academic Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karowe, D.N., Seimens, D.H. & Mitchell-Olds, T. Species-Specific Response of Glucosinolate Content to Elevated Atmospheric CO2 . J Chem Ecol 23, 2569–2582 (1997). https://doi.org/10.1023/B:JOEC.0000006667.81616.18
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000006667.81616.18