Abstract
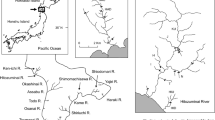
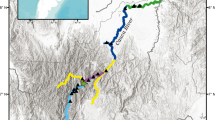
Freshwater fishes that have been isolated by artificial dams have become models for studying the effects of recent barriers on genetic variation and population differentiation. In this study, we examined the genetic structure of 11 populations of white-spotted charr (Salvelinus leucomaenis) by using polymorphic microsatellite loci. Reduced genetic diversity, expressed as the number of alleles and the expected heterozygosity, was observed in all above-dam relative to below-dam populations. Highly significant genetic differentiation (F ST) was found for all pairwise comparisons among populations, with F ST-values ranging from 0.023 to 0.639. Both multiple regression analysis and a randomization test revealed that genetic differentiation above and below dams was negatively related to the habitat size of above-dam populations, and was positively related to the time period of isolation. This study is one of the few attempts to predict the population genetic structure of such variable spatial–temporal scales. We conclude that differences in genetic structure above and below dams are related to recent historical population size, whereby sites with a lower effective number of adults are more prone to temporal stochasticity in gene frequencies.
Similar content being viewed by others
References
Angers B, Bernatchez L, Angers A, Desgroseillers L (1995) Specific microsatellite loci for brook charr reveal strong population subdivision on a microgeographic scale. J. Fish Biol., 47, 177–185.
Angers B, Magnan P, Angers A, Desgroseillers L (1999) Canonical correspondence analysis for estimating spatial and environmental effect on microsatellite gene diversity in brook charr (Salvelinus fontinalis). Mol. Ecol., 8, 1043–1054.
Bachman RA (1984) Foraging behavior of free-living wild and hatchery brown trout in a stream. Trans. Am. Fish. Soc., 113, 1–32.
Bossart JL, Prowell DP (1998) Genetic estimates of population structure and gene flow: limitations, lessons and new directions. Trends Ecol. Evol., 13, 202–206.
Bouza C, Arias J, Castro J, Sanchez L, Martinez P (1999) Genetic structure of brown trout, Salmo trutta L., at the southern limit of the distribution range of the anadromous form. Mol. Ecol., 8, 1991–2001.
Britten HB (1996) Meta-analysis of the association between multilocus heterozygosity and fitness. Evolution, 50, 2158–2164.
Buri P (1956) Gene frequency in small populations of mutant Drosophila. Evolution, 10, 367–402.
Carlsson J, Nilsson J (2000) Population genetic structure of brown trout (Salmo trutta L.) within a northern boreal forest stream. Hereditas, 132, 173–181.
Castric V, Bonney F, Bernatchez L (2001) Landscape structure and hierarchical genetic diversity in the brook charr, Salvelinus fontinalis. Evolution, 55, 1016–1028.
David P (1998) Heterozygosity-tness correlations: new perspectives on old problems. Heredity, 80, 531–537.
Elliott JM (1994) Quantitative Ecology and the Brown Trout. Oxford University Press, Oxford.
Estoup A, Rousset F, Michalakis Y, Cornuet JM, Adriamanga M, Guyomard R (1998) Comparative analysis of microsatellite and allozyme markers: a case study investigating microgeographic di. erentiation in brown trout (Salmo trutta). Mol. Ecol., 7, 339–353.
Falconer DS (1989) Introduction to Quantitative Genetics. Longman Scientific and Technical, London.
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv. Biol., 10, 1500–1508.
Garant D, Dodson JJ, Bernatchez L (2000) Ecological determinants and temporal stability of the within-river population structure in Atlantic salmon (Salmo salar L.). Mol. Ecol., 9, 615–628.
Giles BE, Goudet J (1997) Genetic diffrentiation in Silene dioica metapopulations: estimation of spatiotemporal effects in a successional plant species. Am. Nat., 149, 507–526.
Gore JA (1996) Discharge measurements and streamflow analysis. In: Methods in Stream Ecology (eds. Hauer FR, Lamberti GA), pp. 53–74. Academic Press, California.
Goudet J (1999) PCAGEN ver. 1. 2. Population Genetics Laboratory, University of Lausanne, Lausanne, Switzerland.
Hansen MM, Loeschcke V (1996) Temporal variation in mitochondrial DNA haplotype frequencies in a brown trout (Salmo trutta L.) population that shows stability in nuclear allele frequencies. Evolution, 50, 454–457.
Hansen MM, Mensberg KLD (1998) Genetic differentiation and relationship between genetic and geographical distance in Danish sea trout (Salmo salar L.) populations. Heredity, 81, 493–504.
Hébert C, Danzman RG, Jones MW, Bernatchez L (2000) Hydrography and population genetic structure in brook charr (Salvelinus fontinalis, Mitchill) from eastern Canada. Mol. Ecol., 9, 971–982.
Hedrick PW, Gilpin ME (1997) Genetic effective size of a metapopulation. In: Metapopulation Biology: Ecology, Genetics, and Evolution (eds. Hanski IA, Gilpin ME), pp. 165–181. Academic Press, California.
Hernandez-Martich JD, Smith MH (1997) Downstream gene flow and genetic structure of Gambusia holbrooki (eastern mosquito sh) populations. Heredity, 79, 295–301.
Kimura M, Crow JF (1963) On the maximum avoidance of inbreeding. Gen. Res., 4, 399–415.
Meffe GK, Vrijenhoek RC (1988) Conservation genetics in the management of desert fishes. Conserv. Biol., 2, 157–169.
Morita K, Takashima Y (1998) Effect of female size on fecundity and egg size in white-spotted charr: comparison between sea-run and resident forms. J. Fish Biol., 53, 1140–1142.
Morita K, Yamamoto S (2002) Effects of habitat fragmentation by damming on the persistence of stream-dwelling charr populations. Conserv. Biol., 16, 1318–1323.
Morita K, Yamamoto S, Hoshino N (2000) Extreme life history change of white-spotted char (Salvelinus leucomaenis) after damming. Can. J. Fish. Aquat. Sci., 57, 1300–1306.
Nakano S (1995) Individual differences in resource use, growth and emigration under the influence of a dominance hierarchy in fluvial red-spotted masu salmon in a natural habitat. J. Anim. Ecol., 64, 75–84.
Nakano S, Kitano F, Maekawa K (1996) Potential fragmentation and loss of the thermal habitats for charrs in the Japanese archipelago due to climatic warming. Freshw. Biol., 36, 711–722.
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution, 29, 1–10.
Neraas LP, Spruell P (2001) Fragmentation of riverine systems: the genetic effects of dams on bull trout (Salvelinus confluentus) in the Clark Fork River system. Mol. Ecol., 10, 1153–1164.
Nielsen EE, Hansen MM, Loeschcke V (1999) Genetic variation in time and space: microsatellite analysis of extinct and extant populations of Atlantic salmon. Evolution, 53, 261–268
O'Reilly PT, Hamilton LC, McConnell SK, Wright JM (1996) Rapid analysis of genetic variation in Atlantic salmon (Salmo salar) by PCR multiplexing of dinucleotide and tetranucleotide microsatellites. Can. J. Fish. Aquat. Sci., 53, 2292–2298.
Quattro JM, Vrijenhoek RC (1989) Fitness differences among remnant populations of endangered Sonoran topminnow. Science, 245, 976–978.
Raymond M, Rousset F (1995) GENEPOP (version 3. 1): population genetics software for exact tests and ecumenism. J. Heredity, 86, 248–249.
Rice WR (1989) Analysing tables of statistical tests. Evolution, 43, 223–225.
Schneider S, Roessli D, Excoffier L (2000) ARLEQUIN ver. 2.000: A Software for Population Genetic Data Analysis. Genetics and Biometry Laboratory, Department of Anthropology and Ecology, University of Geneva, Geneva, Switzerland.
Shikano T, Chiyokubo T, Taniguchi N (2001) Temporal changes in allele frequency, genetic variation and inbreeding depression in small populations of the guppy, Poecilia reticulata. Heredity, 86, 153–160.
Shimoda K, Nakano S, Yamamoto S (2002) Landlocking of anadromous white-spotted charr Salvelinus leucomaenis by damming. Jpn. J. Ichthyol., 49, 25–32.
Templeton AR, Robertson RJ, Brisson J, Strasburg J (2001) Disrupting evolutionary process: the effect of habitat fragmentation on collared lizards in the Missouri Ozarks. Proc. Natl. Acad. Sci. USA, 98, 5426–5432.
Tessier N, Bernatchez L (1999) Stability of population structure and genetic diversity across generations assessed by micro-satellites among sympatric populations of landlocked Atlantic salmon (Salmo salar L.). Mol. Ecol., 8, 169–179.
Vrijenhoek RC (1994) Genetic diversity and tness in small populations. In: Conservation Genetics (eds. Loeschcke V, Tomiuk J, Jain SK), pp. 37–53. Birkhäuser Verlag, Basel.
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution, 38, 1358–1370.
Yamamoto S, Nakano S (1996) Growth and development of a bimodal length–frequency distribution during smolting in a wild population of white-spotted charr in northern Japan. J. Fish Biol., 48, 68–79.
Yamamoto S, Morita K, Goto A (1999) Geographic variations in life-history characteristics of white-spotted charr (Salvelinus leucomaenis). Can. J. Zool., 77, 871–878.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamamoto, S., Morita, K., Koizumi, I. et al. Genetic Differentiation of White-Spotted Charr (Salvelinus leucomaenis) Populations After Habitat Fragmentation: Spatial–Temporal Changes in Gene Frequencies. Conservation Genetics 5, 529–538 (2004). https://doi.org/10.1023/B:COGE.0000041029.38961.a0
Issue Date:
DOI: https://doi.org/10.1023/B:COGE.0000041029.38961.a0