Abstract
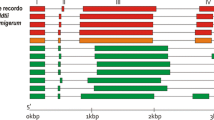
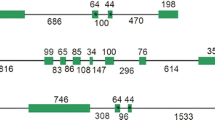
A full-length (LeHT2) and two partial (LeHT1 and LeHT3) cDNA clones, encoding hexose transporters, were isolated from tomato (Lycopersicon esculentum) fruit and flower cDNA libraries. Southern blot analysis confirmed the presence of a gene family of hexose transporters in tomato consisting of at least three members. The full-length cDNA (LeHT2) encodes a protein of 523 amino acids, with a calculated molecular mass of 57.6 kDa. The predicted protein has 12 putative membrane-spanning domains and belongs to the Major Facilitator Superfamily of membrane carriers. The three clones encode polypeptides that are homologous to other plant monosaccharide transporters and contain conserved amino acid motifs characteristic of this superfamily. Expression of the three genes in different organs of tomato was investigated by quantitative PCR. LeHT1 and LeHT3 are expressed predominantly in sink tissues, with both genes showing highest expression in young fruit and root tips. LeHT2 is expressed at relatively high levels in source leaves and certain sink tissues such as flowers. LeHT2 was functionally expressed in a hexose transport-deficient mutant (RE700A) of Saccharomyces cerevisiae. LeHT2-dependent transport of glucose in RE700A exhibited properties consistent with the operation of an energy-coupled transporter and probably a H+/hexose symporter. The K m of the symporter for glucose is 45 μM.
Similar content being viewed by others
References
Asano, T., Katagiri, H., Takata, K., Lin, J.L., Ishihara, H., Inukai, K., Tsukuda, K., Kikuchi, M., Hirano, H., Yazaki, Y. and Oka, Y. 1991. The role of N-glycosylation of GLUT1 for glucose transport activity. J. Biol. Chem. 266: 24632–24636.
Boorer, K.J., Loo, D.D.F. and Wright, E.M. 1994. Steady-state and presteady-state kinetics of the HC/hexose cotransporter (STP1) from Arabidopsis thaliana expressed in Xenopus oocytes. J. Biol. Chem. 269: 20417–20424.
Bugos, R.C. and Thom, M. 1993. Glucose transporter cDNAs from sugarcane. Plant Physiol. 103: 1469–1470. Bush, D.R. 1993. Proton-coupled sugar and amino acid transporters in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44: 513– 542.
Büttner, M., Truernit, E., Baier, K., Scholz-Starke, J., Sontheim, M., Lauterbach, C., Huss, V.R. and Sauer, N. 2000. AtSTP3, a green leaf-specific, low affinity monosaccharide-HC symporter of Arabidopsis thaliana. Plant Cell Environ. 23: 175–184.
Caspari, T., Robl, I., Stolz, J. and Tanner, W. 1996. Purification of the Chlorella HUP1 hexose-proton symporter to homogeneity and its reconstitution in vitro. Plant J. 10: 1045–1053.
Elble, R. 1992. A simple and efficient procedure for transformation of yeasts. Biotechniques 13: 18–20.
Felle, H., Gogarten, J.P. and Bentrup, F.W. 1983. Phlorizin inhibits hexose transport across the plasmalemma of Riccia fluitans. Planta 157: 267–270.
Getz, H.P., Knauer, D. and Willenbrink, J. 1987. Transport of sugar across the plasma membrane of beetroot protoplasts. Planta 171: 185–196.
Gogarten, J.P. and Bentrup, F.W. 1989. Substrate specificity of the hexose carrier in the plasmalemma of Chenopodium rubrum suspension cells probed by transmembrane exchange diffusion. Planta 178: 52–60.
Harrison, M.J. 1996. A sugar transporter from Medicago truncatula: altered expression pattern in roots during vesicular-arbuscular (VA) mycorrhizal associations. Plant J. 9: 491–503.
Kephart, D. 1998. Quantitative RT-PCR: rapid construction of templates for competitive amplification. Promega Notes 68: 16–19.
Köhler, T. 1995. General aspects and chances of nucleic acid quantitation by PCR. In: T. Köhler, D. Lassner, A.-K. Rost, B. Thamm, B. Pustowoit and H. Remke (Eds) Quantitation of mRNA by Polymerase Chain Reaction, Springer-Verlag, Berlin, pp. 3–14.
Lalonde, S., Boles, E., Hellmann, H., Barker, L., Patrick, J.W., Frommer, W.B. and Ward, J.M. 1999. The dual function of sugar carriers: transport and sugar sensing. Plant Cell 11: 707–726.
Lashbrook, C.C., Gonzalez-Bosch, C. and Bennett, A.B. 1994. Two divergent endo-β-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell 6: 1485–1493.
Lemoine, R. and Delrot, S. 1987. Recognition of phlorizin by the carriers of sucrose and hexose in broad bean leaves. Physiol. Plant. 69: 639–644.
Lin, W., Schmitt, M.R., Hitz, W.D. and Giaquinta, R.T. 1984. Sugar transport in isolated corn root protoplasts. Plant Physiol. 76: 894–897.
Loison, G. 1994. Production of foreign proteins at high level. In: J.R. Johnston (Ed.) Molecular Genetics of Yeast: A Practical Approach, Oxford University Press, Oxford, pp. 161–180.
Maiden, M.C.J., Davis, E.O., Baldwin, S.A., Moore, D.C.M. and Henderson, P.J.F. 1987. Mammalian and bacterial sugar transport proteins are homologous. Nature 325: 641–643.
Marger, M.D. and Saier, M.H. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 18: 13–20.
Martinoia, E., Kaiser, G., Schramm, M.J. and Heber, U. 1987. Sugar transport across the plasmalemma and the tonoplast of barley mesophyll protoplasts. Evidence for different transport systems. J. Plant Physiol. 131: 467–478.
Maynard, J.W. and Lucas, W.J. 1982. Sucrose and glucose uptake into Beta vulgaris leaf tissues. A case for general (apoplastic) retrieval systems. Plant Physiol. 70: 1436–1443.
Opekarova, M., Caspari, T. and Tanner, W. 1994. The HUP1 gene product of Chlorella kessleri: HC/glucose symport studied in vitro. Biochim. Biophys. Acta 1194: 149–154.
Reifenberger, E., Freidel, K. and Ciriacy, M. 1995. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol. Microbiol. 16: 157–167.
Rentsch, D., Boorer, K.J. and Frommer, W.B. 1998. Structure and function of plasma membrane amino acid, oligopeptide and sucrose transporters from higher plants. J. Membr. Biol. 162: 177–190.
Roitsch, T. and Tanner, W. 1994. Expression of a sugartransporter gene family in a photoautotrophic suspension culture of Chenopodium rubrum L. Planta 193: 365–371.
Ruan, Y.L. and Patrick, J.W. 1995. The cellular pathway of postphloem sugar transport in developing tomato fruit. Planta 196: 434–444.
Ruan, Y.L., Patrick, J.W. and Brady, C. 1997. Protoplast hexose carrier activity is a determinate of genotypic difference in hexose storage in tomato fruit. Plant Cell Environ. 20: 341–349.
Sauer, N. and Stadler, R. 1993. A sink-specific HC/monosaccharide co-transporter from Nicotiana tabacum: cloning and heterologous expression in baker's yeast. Plant J. 4: 601–610.
Sauer, N., Caspari, T., Klebl, F. and Tanner, W. 1990a. Functional expression of the Chlorella hexose transporter in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 87: 7949–7952.
Sauer, N., Friedlander, K. and Graml Wicke, U. 1990b. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J. 9: 3045–3050.
Sauer, N., Baier, K., Gahrtz, M., Stadler, R., Stolz, J. and Truernit, E. 1994. Sugar transport across the plasma membranes of higher plants. Plant Mol. Biol. 26: 1671–1679.
Smith, F.W., Ealing, P.M., Hawkesford, M.J. and Clarkson, D.T. 1995. Plant members of a family of sulfate transporters reveal functional subtypes. Proc. Natl. Acad. Sci. USA 92: 9373–9377.
Stadler, R., Wolf, K., Hilgarth, C., Tanner, W. and Sauer, N. 1995. Subcellular localization of the inducible Chlorella HUP1 monosaccharide-HC symporter and cloning of a co-induced galactose-HC symporter. Plant Physiol. 107: 33–41.
Stanzel, M., Sjölund, R.D. and Komor, E. 1988. Transport of glucose, fructose and sucrose by Streptanthus tortuosus suspension cells. I. Uptake at low sugar concentration. Planta 174: 201–209.
Tanner, W. and Caspari, T. 1996. Membrane transport carriers. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 595–626.
Truernit, E., Schmid, J., Epple, P., Illig, J. and Sauer, N. 1996. The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8: 2169–2182.
Truernit, E., Stadler, R., Baier, K. and Sauer, N. 1999. A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J. 17: 191–201.
Tubbe, A. and Buckhout, T.J. 1992. In vitro analysis of the HChexose symporter on the plasma membrane of sugarbeets (Beta vulgaris L.). Plant Physiol. 99: 945–951.
Verstappen, R., Ranostaj, S. and Rausch, T. 1991. The hexose transporters at the plasma membrane and the tonoplast of transformed plant cells: kinetic characterization of two distinct carriers. Biochim. Biophys. Acta 1073: 366–373.
Weber, H., Borisjuk, L., Heim, U., Sauer, N. and Wobus, U. 1997. A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell 9: 895–908.
Weig, A., Franz, J., Sauer, N. and Komor, E. 1994. Isolation of a family of cDNA clones from Ricinus communis L. with close homology to the hexose carriers. J. Plant Physiol. 143: 178–183.
Will, A., Caspari, T. and Tanner, W. 1994. Km mutants of the Chlorella monosaccharide/HC cotransporter randomly generated by PCR. Proc. Natl. Acad. Sci. USA 91: 10163–10167.
Xia, J.H. and Saglio, P.H. 1988. Characterization of the hexose transport system in maize root tips. Plant Physiol. 88: 1015– 1020.
Ylstra, B., Garrido, D., Busscher, J. and van Tunen, A.J. 1998. Hexose transport in growing petunia pollen tubes and characterization of a pollen-specific, putative monosaccharide transporter. Plant Physiol. 118: 297–304.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gear, M.L., McPhillips, M.L., Patrick, J.W. et al. Hexose transporters of tomato: molecular cloning, expression analysis and functional characterization. Plant Mol Biol 44, 687–697 (2000). https://doi.org/10.1023/A:1026578506625
Issue Date:
DOI: https://doi.org/10.1023/A:1026578506625