Abstract
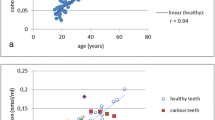
Mature tooth dentin has essentially no metabolic activity, and thus post-translational modifications accumulate with aging in this tissue. In the present paper, we have studied age-related covalent changes of human dentin proteins. Dentin phosphoproteins (PP) were extracted and purified using ion exchange chromatography. Collagen was purified by CNBr cleavage and acetic acid extraction. The amino acid composition of the resultant protein preparations was determined by HPLC after post-column derivatization. Likewise the extent of aspartic acid (Asp) racemization was determined in total dentin, dentin collagen and PP. Collagen only displayed small, insignificant changes in amino acid composition and racemization with age. In contrast, PP exhibited significant age-related changes in amino acid composition, cross-linking and racemization. Thus the rate of Asp racemization in PP was 500-fold that found in collagen. Moreover, the phosphoserine (Ser(P)) content in human PP decreases dramatically with age, resulting in almost complete dephosphorylation over a life span. The loss of Ser(P) was accompanied by an increased content of the bifunctional cross-link histidinoalanine consistent with a β-elimination pathway. The relative Ser(P) content was highly correlated with dentin age (r2 = 0.96). The Ser(P) contents may thus potentially be applied in forensic investigations to deduce human age. The possible role of covalent modifications of the protein matrix in the degradation of mineralized tissue and its implications for the age-related decline of tissue functionality is discussed.
Similar content being viewed by others
References
Addadi L and Weiner S (1985) Interactions between acidic proteins and crystalls stereochemical requirements in biomineralization. Proc Natl Acad Sci USA 82: 4110–4114
Aswad DW (1984) Determination of D-and L-aspartate in amino acid mixtures by high-performance chromatography after derivatization with a chiral adduct of O-phthaldialdehyde. Anal Biochem 137: 405–409
Barkholt V and Jensen AL (1989) Amino acid analysis: Determination of cysteine plus half-cysteinein proteins after hydrochloric acid hydrolysis with a disulfide compound as additive. Anal Biochem 177: 318–322
Bonde M, Qvist P, Fledelius C, Riis BJ and Christiansen C (1994) Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin Chem 40: 2022–2025
Bonde M, Fledelius C, Qvist P and Christiansen C (1996) CoatedtuberRadioimmunoassay for C-telopeptides of type I collagen to assess bone resorption. Clin Chem 42: 1639–1644
Borggreven JMPM, Hoppenbrouwers PMM and Gorissen R (1979) Radiochemical determination of the metabolic activity of collagen in mature dentin. J Dent Res 58: 2120–2124
Bylund DB and Huang TS (1976) Decomposition of Phosphoserine and phosphothreonine during acid hydrolysis. Anal Biochem 73: 477–485
Brady JD, Ju J and Robins SP (1999) Isoaspartyl Bond formation within N-terminal sequences of collagen type I: implications for their use as markers of collagen degradation. Clinical Science 96: 209–215
Butler WT (1998) Dentin matrix proteins. Eur J Oral Sci 106(Suppl 1): 204–210
Cloos PAC and Fledelius C (2000) Collagen fragments in urine derived from bone resorption are highly racemized and isomerized. A biological clock of protein ageing with clinical potential. Biochem J 345: 473–480
Fisher LW and Termine JD (1985) Purification of the Noncollagenous proteins from bone; technical pitfalls and how to avoid them. InL Ornoy A, Harell A and Sela J (eds) Current Advances in Skeletogenesis, pp 467–472. Elsevier Science.
Fledelius C, Johnsen AH, Cloos PAC, Bonde M and Qvist P (1997) Characterization of urinary degradation products derived from type I collagen. J Biol Chem 272: 9755–9763
Fujisawa R, Kuboki Y and Sasaki S (1985) In vivo cleavage of dentin phosphophoryn following β-elimination of its phosphoserine residues. Archs Biochem Biophys 243: 619–623
Fujisawa R, Kuboki Y and Sasaki S (1986) Changes in interaction of bovine dentin phosphophoryn with calcium and hydroxyapatite by chemical modifications. Calcif Tissue Int 39: 248–251
Geiger T and Clarke S (1987) Deamidation, isomerization, and racemization at asparginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem 262: 785–794
Gorsky JP (1992) Acid phosphoproteins from bone matrix: A structural rationalization of their role in biomineralization. Calcif Tissue Int 50: 391–396
Helfman PM and Bada JL (1976) Aspartic acid racemization in dentine as a measure of aging. Nature, 262: 279–281
Henle T, Walter AW and Klostermeyer H (1993) Detection and identification of the cross-linking amino acids Nτ and Nπ-(2′-amino-2′-carboxy-ethyl)-L-histidine ('histidinoalanine', HAL) in heated milk products. Z Lebensm Unters Forsch 197: 114–117
Hol WGJ, Halie LM and Sander C (1981) Dipoles of the α-helix and β-sheet their role in protein folding. Nature 294: 532–536
Jensen AL and Foltmann B (1996) Determination of phosphoserine in human pepsinogens. Scand J Clin Lab Invest 56: 69–74
Jones YE and Miller A (1987) Structural models for the N-and C-terminal telopeptide regions of interstitial collagens. Biopolymers 26: 463–480
Jontell M, Pertoft R and Linde A (1982) Disagreement in molecular weight determinations of dentin phosphoprotein. Biochim Biophys Acta 705: 315–320
Kuboki Y, Fujisawa R, Tsuzaki M, Liu CF and Sasaki S (1984) Presence of lysinoalanine and histidinoalanine in bovine dentin phosphoprotein. Calcif Tissue Int 36: 126–128
Lee SL, Kossiva D and Glimcher MJ (1983) Phosphoproteins from bovine dentin: evidence for polydispersity during tooth maturation. Biochemistry 22: 2596–2601
Lowenstam HA and Weiner S (1989) in Lowenstam HA and Weiner S (eds) On Biominineralization, pp 25–44. Oxford University Press, New York
MacDougall M, Simmons D, Luan X, Nydegger J, Feng J and Gu TT (1997) Dentin Phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. J Biol Chem 272: 835–842
Maggiora GM, Mao B and Chou K-C (1991) Chiral features of proteins. In: Mezey PG (ed) New Developments in Molecular Chirality, pp 93–118. Kluwer Academic Publishers, Dordrecht, The Netherlands
Masters PM (1983) Stereochemically altered noncollagenous proteins of human dentin Calcif Tissue Int 35: 43–47
Masters PM (1985) In vivo decomposition of phosphoserine and serine in non-collagenous proteins from human dentin. Calcif Tissue Int 37: 236–241
McCurdy SP, Clarkson BH, Speirs RL and Feagin FF (1990) Phosphoprotein extraction from the dentine/cementum complex of human tooth roots. Archs Oral Biol 35: 347–357
McCurdy SP, Clarckson BH and Feagin FF (1992) Comparison of phosphoprotein isolated from mature and immature human tooth roots. Archs Oral Biol 37: 1057–1065
Moradian-Oldak J, Frolow F, Addadi L and Weiner S (1992) Interaction between acidic macromolecules and calcium phosphate ester crystalls: relevance to carbonate formation in biomineralization. Proc R Soc Lond B 247: 47–55
Neuberger A (1948) Stereochemistry of amino acids. Adv Protein Chem 4: 298–383
Ogino T, Ogiuchi H, Hobo T and Ogino H (1988) Effect of acid hydrolysis on the racemization method using amino acid racemization in teeth. Bunseki Kagaku 37: 115–117
Ohtani S and Yamamoto K (1992) Estimation of age from a tooth by means of racemization of an amino acid, especially aspartic acid – comparison of enamel and dentin. J Forensic Sci 37: 1061–1067
Patchorkin A and Sokolowski MJ (1964) Nonenzymatic cleavages of peptide chains at the cysteine and serine residues through their conversion into dehydroalanine. Amer Chem Soc 86: 1206–1212
Radkiewicz JL, Zipse H, Clarke S and Houk KN (1996) Accelerated racemization of aspartic acid and asparagine residues via succinimide intermediates: an ab initio theoretical exploration of mechanism. J Am Chem Soc 118: 9148–9155
Rahima M and Veis A (1988) Two classes of dentin phosphophoryns, from a wide range of species, containing immunologically cross-reactive epitope regions. Calcif Tissue Int 42: 104–112
Robey PG (1996) Vertebrate mineralized matrix proteins: structure and function. Connect Tissue Res 35: 131–136
Rosenstein RW and Taborsky G (1970) Mechanism of the oxidative dephosphorylation phosphoprotein phosvitin. Biochem 9: 649–657
Saito T, Arsenault AL, Yamauchi M, Kuboki Y and Crenshaw MA (1997) Mineral induction by immobilized phosphoprotein. Bone 21: 305–311
Saleh N, Deutsch D and Gil-Av E (1993) Racemization of aspartic acid in the extracellular matrix proteins of primary and secondary dentin. Calcif Tissue Int 53: 103–110
Schroeder RA and Bada JL (1977) Kinetics and mechanisms of the epimerization and decomposition of threonine in fossil foraminifera. Geochim Cosmochimica Acta 41: 1087–1095
Scott PG and Veis A (1976) The cyanogen bromide peptides of bovine soluble and insoluble collagens. Part I–II. Conn Tissue Res 4: 107–129
Shah NK, Brodsky B, Kirkpatrick A and Ramshaw JAM (1999) Structural consequences of d-amino acids in collagen triplehelical peptides. Biopolymers 49: 297–302
Shikata H, Hiramatsu M, Masumizu T, Fujimoto D and Utsumi N (1985) Age-related changes in the content of non-reducible cross-links in rat mandibular bone. Archs Oral Biol 30: 451–453
Smith GG and Evans RC (1980) The effect of structure and conditions on the rate of racemization of free and bound amino acids. In: Hare PE, Hoering TC and King C Jr (eds) Biogeochemistry of Amino Acids, pp 257–282. John Wiley, New York
Szendrei GI, Fabian H, Mantsch HH, Lovas SN, Nyeki O, Schon I and Otvos L (1994) Aspartate-bond isomerization affects the major conformations of synthetic peptides. Eur J Biochem 226: 917–924
Takagi Y and Veis A (1984) Isolation of phosphophoryn from human dentin organic matrix. Calcif Tissue Int 36: 259–265
Tyler-Cross R and Schirch V (1991) Effects of amino acid sequence, buffers, and ionic strength on the rate and mechanism of deamidation of asparagine residues in small peptides. J Biol Chem 266: 22549–22556
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cloos, P.A., Jensen, A.L. Age-related de-phosporylation of proteins in dentin: A biological tool for assessment of protein age. Biogerontology 1, 341–356 (2000). https://doi.org/10.1023/A:1026534400435
Issue Date:
DOI: https://doi.org/10.1023/A:1026534400435