Abstract
Purpose. Although the rate of drug release from poly(D,L-lactide-co-glycolide) (PLG) microspheres is often modulated by changing fabrication conditions or materials, the specific factors directly controlling the release profiles are often unclear. We have fabricated uniform rhodamine- and piroxicam-containing microspheres, 10 to 100 μm in diameter, to better understand how microsphere size controls drug release.
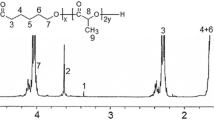
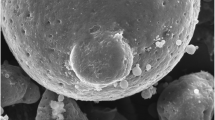
Methods. Drug distribution within the microspheres was examined using confocal fluorescence microscopy. The rate of polymer degradation was determined as the change in molecular weight, measured by gel permeation chromatography, during in vitro degradation experiments. Further, changes in the surface and interior morphology of the particles during in vitro degradation were investigated by scanning electron microscopy.
Results. Microsphere size greatly affected drug distribution. Small (∼10-μm) microspheres showed an essentially uniform drug distribution. Larger (∼100-μm) microspheres showed redistribution of drug to specific regions of the microspheres. Rhodamine partitioned to the surface and piroxicam partitioned to the interior of large PLG microspheres. Further, the rate of polymer degradation increased with microsphere size, possibly the result of a more acidic interior caused by increased accumulation of hydrolyzed polymer products in larger particles. Finally, larger microspheres developed a more porous interior structure during the drug release.
Conclusions. Microsphere size affects drug release not only through changes in diffusion rates but also through secondary effects including drug distribution in the particle, polymer degradation rate, and microsphere erosion rates.
Similar content being viewed by others
REFERENCES
J. Akbuga. Effect of microsphere size and formulation factors on drug release from controlled-release furosemide microspheres. Drug Dev. Ind. Pharm. 17:593–607 (1991).
S. Cohen, T. Yoshika, M. Lucarelli, L. H. Hwang, and R. Langer. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 8:713–720 (1991).
P. Sansdrap and A. J. Moes. In vitro evaluation of the hydrolytic degradation of dispersed and aggregated poly(dl-lactide-co-glycolide) microspheres. J. Control. Release 43:47–58 (1997).
R. Narayani and K. Panduranga Rao. Gelatin microsphere cocktails of different sizes for the controlled release of anticancer drugs. Int. J. Pharm. 143:255–258 (1996).
J. M. Bezemer, R. Radersma, D. W. Grijpma, P. J. Dijkstra, C. A. van Blitterswijk, and J. Feijen. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers 2. Modulation of release rate. J. Control. Release 67:249–260 (2000).
M. Tracy, K. Ward, L. Firouzabadian, Y. Wang, N. Dong, R. Qian, and Y. Zhang. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 20:1057–1062 (1999).
Y. Men, C. Thomasin, H. P. Merkle, B. Gander, and G. Corradin. A single administration of tetanus toxoid in biodegradable microspheres elicits T cell and antibody responses similar or superior to those obtained with aluminum hydroxide. Vaccine 13:683–689 (1995).
M. Sandor, D. Enscore, P. Weston, and E. Mathiowitz. Effect of protein molecular weight on release from micron sized PLGA microspheres. J. Control. Release 76:297–311 (2001).
Y.-Y. Yang, T.-S. Chung, and N. P. Ng. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 22:231–241 (2001).
A. G. A. Coombes, Y. Ming-Kung, E. C. Lavelle, and S. S. Davis. The control of protein release from poly(dl-lactide co-glycolide) microparticles by variation of the external aqueous phase surfactant in the water-in oil-in water method. J. Control. Release 52:311–320 (1998).
J. M. Anderson and M. S. Shive. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 28:5–24 (1997).
K. Fu, D. W. Pack, A. M. Klibanov, and R. Langer. Visual evidence of acidic environment within degrading PLGA microspheres. Pharm. Res. 17:100–106 (2000).
M. Dunne, O. I. Corrigan, and Z. Ramtolla. Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials 21:1659–1668 (2000).
C. Berkland, K. Kim, and D. W. Pack. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J. Control. Release 73:59–74 (2001).
C. Berkland, M. King, A. Cox, K. Kim, and D. Pack. Precise control of PLG microsphere size provides enhanced control of drug release rate. J. Control. Release 82:137–147 (2002).
C. Berkland, K. Kim, and D. Pack. Precision polymer microparticles for controlled-release drug delivery, Carrier-Based Drug Delivery, ACS Symposium Series (2002); Orlando, Florida.
J. Wang, B. M. Wang, and S. P. Schwendeman. Characterization of the initial burst release of a model peptide from poly(dl-lactide-co-glycolide) microspheres. J. Control. Release 82:289–307 (2002).
N. Faisant, J. Siepmann, and J. P. Benoit. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur. J. Pharm. Sci. 15:355–366 (2002).
R. P. Batycky, J. Hanes, R. Langer, and D. A. Edwards. A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. J. Pharm. Sci. 86:1464–1477 (1997).
W. M. Saltzman and R. Langer. Transport rates of proteins in porous polymers with known microgeometry. Biophys. J. 55:163–171 (1989).
W. M. Saltzman, S. H. Pasternak, and R. Langer. Microstructural models for diffusive transport in porous polymers. ACS Symposium Series 348 (1987) 16–33.
J. Hadgraft, J. du Plessis, and C. Goosen. The selection of non-steroidal anti-inflammatory agents for dermal delivery. Int. J. Pharm. 207:31–37 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berkland, C., Kim, K.(. & Pack, D.W. PLG Microsphere Size Controls Drug Release Rate Through Several Competing Factors. Pharm Res 20, 1055–1062 (2003). https://doi.org/10.1023/A:1024466407849
Issue Date:
DOI: https://doi.org/10.1023/A:1024466407849