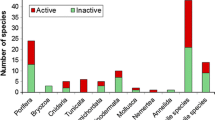
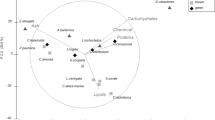
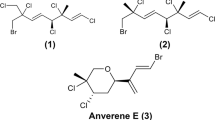
Abstract
Accurate knowledge of factors affecting the survival of early life stages of marine invertebrates is critically important for understanding their population dynamics and the evolution of their diverse reproductive and life-history characteristics. Chemical defense is an important determinant of survival for adult stages of many sessile benthic invertebrates, yet relatively little consideration has been given to chemical defenses at the early life stages. This review examines the taxonomic breadth of early life-stage chemical defense in relation to various life-history and reproductive characteristics, as well as possible constraints on the expression of chemical defense at certain life stages. Data on the localization of defensive secondary metabolites in larvae and the fitness-related consequences of consuming even a small amount of toxic secondary metabolites underpin proposals regarding the potential for Müllerian and Batesian mimicry to occur among marine larvae. The involvement of microbial symbionts in the chemical defense of early life stages illustrates its complexity for some species. As our knowledge of chemical defenses in early life stages grows, we will be able to more rigorously examine connections among phylogeny, chemical defenses, and the evolution of reproductive and life-history characteristics among marine invertebrates.
Similar content being viewed by others
REFERENCES
Augner, M. and Bernays, E. A. 1998. Plant defense signals and Batesian mimicry. Evol. Ecol. 12:667–679.
Benkendorff, K., Bremner, J. B., and Davis, A. R. 2000. Tyrian purple precursors in the egg masses of the Australian muricid Dicathais orbita: a possible defensive role. J. Chem. Ecol. 26:1037–1050.
Bullard, S. G., Lindquist, N. L., and Hay, M. E. 1999. Susceptibility of invertebrate larvae to predators: how common are post-capture larval defenses? Mar. Ecol. Prog. Ser. 191:153–161.
Coll, J. C., Leone, P. A., Bowden, B. F., Carroll, A. R., KÖnig, G. M., Heaton, A., De Nys, R., Maida, M., AliÑo, P. M., Willis, R. H., Babcock, R. C., Florian, Z., Clayton, M. N., Miller, R. L., and Alderslade, P. N. 1995. Chemical aspects of mass spawning in corals. II. (°)-epi-Thunbergol, the sperm attractant in the eggs of the soft coral Lobophytum crassum (Cnidaria: Octocorallia). Mar. Biol. 123:137–143.
Cowart, J. D., Fielman, K. T., Woodin, S. A., and Lincoln, D. E. 2000. Halogenated metabolites in two marine polychaetes and their planktotrophic and lecithotrophic larvae. Mar. Biol. 136:993–1002.
Cowden, C., Young, C., M., and Chia., F.-S. 1984. Differential predation on marine invertebrate larvae by two benthic predators. Mar. Ecol. Prog. Ser. 14:145–149.
Davidson, S. K., Allen, S. W., Lim, G. E., Anderson, C. M., and Haygood, M. G. 2001. Evidence for the biosynthesis of bryostatins by the bacterial symbiont “Candidatus Endobugula sertula” of the bryozoan Bugula neritina. Appl. Environ Micro 67:4531–4537.
Dunlap, M. and Pawlik, J. R. 1996. Video-monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges. Mar. Biol. 126; 117–123.
Faulkner, D. J. 2002. Marine natural products. J. Nat. prod. 19:1–48.
Faulkner, D. J., Harper, M. K., Salomon, C. E., and Schmidt, E.W. 1999. Localisation of bioactive metabolites in marine sponges. Mem. Queensl. Mus. 44:167–173.
Gil-Turnes, M. S. and Fenical, W. 1992. Embryos of Homarus americanus are protected by epibiotic bacteria. Biol. Bull. 182:105–108.
Gil-Turnes, M. S., Hay, M. E., and Fenical, W. 1989. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 246:116–118.
Gosselin, L. A. and Qian, P.-Y. 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146:256–282.
Grosberg, R. K. and Quinn, J. F. 1986. The genetic control and consequences of kin recognition by the larvae of a colonial marine invertebrate. Nature 322:456–459.
Harvell, C. D., West, J. M., and Griggs, C. 1996. Chemical defense of embryos and larvae of a West Indian gorgonian coral, Briareum asbestinus. lnven. Reprod. Dev. 30:239–246. CHEMICAL DEFENSE OF EARLY LIFE-STAGE INVERTEBRATES 1999
Hay, M. E. 1996. Marine chemical ecology: what's known and what's next? J. Exp. Mar. Biol. Ecol. 200:103–134.
Haygood, M. G., Schmidt, E.W., Davidson, S. K., and Faulkner, D. J. 1999. Microbial symbionts of marine invertebrates; opportunities for microbial biotechnology. J. Mol. Microbiol. Biotechnol. 1:33–43.
Howden, M.E.H., Lucas, J. S., McDuff, M., and Salathe, R. 1975. Chemical defense of Acanthaster planci, pp. 67–79, in Crown-of-thorns Starfish Seminar Proceedings, Brisbane 1974. Australian Govt. Publ. Service, Canberra.
Hunt, H. L. and Scheibling, R. E. 1997. Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Mar. Ecol. Prog. Ser. 155:269–301.
Iyengar, E. V. and Harvell, C. D. 2001. Predator deterrence of early developmental stages of temperate lecithotrophic asteroids and holothuroids. J. Exp. Mar. Biol. Ecol. 264:171–188.
Jackson, J. B. C. 1985. Distribution and ecology of clonal and aclonal benthic invertebrates, pp. 297–355 in J. B. C. Jackson, L. W. Buss, and R. E. Cook (eds.). Population Biology and Evolution of Clonal Organisms. Yale University Press, New Haven Connecticut.
Jackson, J. B. C. 1986. Modes of dispersal of clonal benthic invertebrates: consequences for species distributions and genetic population structure of local population. Bull. Mar. Sci. 39:588–606.
Janzen, D. H. 1971. Seed predation by animals. Annu. Rev. Ecol. Syst. 2:465–492.
Kelman, D., Kushmaro, A., Loya, Y., Kashman, Y., and Benayahu, Y. 1998. Antimicrobial activity of a Red Sea soft coral, Parerythropodium fulvum fulvum: reproductive and developmental considerations. Mar. Ecol. Prog. Ser. 169:87–95.
Klumpp, D. W., McKinnon, A. D., and Mundy, C. N. 1988. Motile cryptofauna of a coral reef: abundance, distribution and trophic potential. Mar. Ecol. Prog. Ser. 45: 95–108.
Kobayashi, J. and Ishibashi, M. 1993. Bioactive metabolites of symbiotic marine microorganisms. Chem. Rev. 93:1753–1769.
Lasker, H. R., Kim, K., and Coffroth, M. A. 1998. Production, settlement, and survival of plexaurid gorgonian recruits. Mar. Ecol. Prog. Ser. 162:111–123.
Lindquist, N. 1996. Palatability of invertebrate larvae to corals and sea anemones. Mar. Biol. 126:745–755.
Lindquist, N. and Fenical, W. 1991. New tambjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators-origin of the tambjamines in Atapozoa. Experientia 47:504–506.
Lindquist, N. and Hay, M. E. 1995. Can small rare prey be chemically defended? The case for marine larvae. Ecology 76:1347–1358.
Lindquist, N. and Hay, M. E. 1996. Palatability and chemical defense of marine invertebrate larvae. Ecol. Monogr. 66:431–450.
Lindquist, N., Hay, M. E., and Fenical, W. 1992. Defense of ascidians and their conspicuous larvae: adult vs. larval chemical defenses. Ecol. Monogr. 62:547–568.
Lindquist, N., Bolser, R., and Laing, K. 1997. Timing of larval release by two Caribbean demosponges. Mar. Ecol. Prog. Ser. 155:309–313.
Lucas, J. S., Hart, R. J., Howden, M. E., and Salathe, R. 1979. Saponins in eggs and larvae of Acanthaster planci (L.) (Asteroidea) as chemical defenses against planktivorous fish. J. Exp. Mar. Biol. Ecol. 40:155–165.
Luckenbach, M. W. and Orth, R. J. 1990. A chemical defense in Crustacea? J. Exp. Mar. Bio. Ecol. 137:79–87.
Maldonado, M. and Uriz, M. J. 1998. Microrefuge exploitation by subtidal encrusting sponges: patterns of settlement and post-settlement survival. Mar. Ecol. Prog. Ser. 174:141–130.
Martin, D., Nourichel, C. L., Uriz, M. J., Bhaud, M., and Duch \(e \circ\) ne, J. C. 2000. Ontogenic shifts in chemical defenses of the northwest Mediterranean Sea Eupolymnia nebulosa (Polychaeta, Terebellidae). Bull. Mar. Sci. 67:287–298.
McEdward, L. R. 1997. Reproductive strategies of marine benthic invertebrates revisited: facultative feeding by planktotrophic larvae. Am. Nat. 150:48–72.
McClintock, J. B. and Baker, B. J. 1997. Palatability and chemical defense of eggs, embryos and larvae of shallow-water Antarctic marine invertebrates. Mar. Ecol. Prog. Ser. 154:121–131.
McClintock, J. B. and Baker, B. J. (eds.). 2001. Marine Chemical Ecology. CRC Press, Boca Raton, Florida.
McClintock, J. B. and Vernon, J. D. 1990. Chemical defense in the eggs and embryos of Antarctic sea stars (Echinodermata). Mar. Biol. 105:4910–495.
McClintock, J. B., Baker, B. J., and Steinberg, D. K. 2001. The chemical ecology of invertebrate meroplankton and holoplankton, pp. 195–225 in J. B. McClintock and B. J. Baker (eds.). CRC Press, Boca Raton, Florida. Marine Chemical Ecology.
Morgan, S. G. 1995. Life and death in the plankton: larval mortality and adaptation, pp. 279–321, in L. R. McEdwards (e.d.). Ecology of Marine Invertebrate Larvae. CRC Press, Boca Raton, Florida.
Orians, G. H. and Janzen, D. H. 1974. Why are embryos so tasty? Am. Nat. 108:581–592.
Osman, R. W. and Whitlatch, R. B., 1995. Predation on early ontogenetic life stages and its effect on recruitment into a marine epifaunal community. Mar. Ecol. Prog. Ser. 117:111–126.
Paul, V. J. 1992. Ecological Roles of Marine Natural Products. Comstock Publishing Associates, Ithaca, New York, 245, p.
Pawlik, J. R. 1993. Marine invertebrate chemical defenses. Chem. Rev. 93:1911–1922.
Provenza, F. D., Kimball, and B. A., Villalba, J. J. 2000. Roles of odor, taste, and toxicity in the food preferences of lambs: implications for mimicry in plants. Oikos 88:424–432.
Roper, T. J. and Marples, N. M. 1997. Odour and colour as cues for taste-avoidance learning in domestic chicks. Anim. Behav. 53:1241–1250.
Ruppert, E. E. and Barnes, R. D. 1994. Invertebrate Zoology. Saunders College Publishing, Phildelphia, Pennsylvania.
Schimtt, T. M., Hay, M. E., and Lindquist, N. 1995. Constraints on chemically mediated coevolution: multiple functions for seaweed secondary metabolites. Ecology 76: 107–123.
Slattery, M., Hines, G. A., Starmer, J., and Paul, V. J. 1999. Chemical signals in gametogenesis, spawning, and larval settlement and defense of the soft coral Sinularia polydactyla. Coral Reefs 18:75–84.
Stachowicz, J. J. and Lindquist., N. 1997. Chemical defense among hydroids on pelagic Sargassum: predator deterrence and absorption of solar UV radiation by secondary metabolites. Mar. Ecol. Prog. Ser. 155:115–126.
Swihart, R. K. and Bryant, J. P. 2001. Importance of biogeography and ontogeny of woody plants in winter herbivory by mammals. J. Mammal. 82:1–21.
Uriz, M. J., Turon, X., Becerro, M. A., and Galera, J. 1996. Feeding deterrence in sponges. The role of toxicity, physical defenses, energetic contents, and life-history stage. J. Exp. Mar. Biol. Ecol. 205:187–204.
Vance, R. R. 1973. On reproductive strategies in marine benthic invertebrates. Am. Nat. 107:339–352.
Young, C. M. and Bingham, B. L. 1987. Chemical defense and aposematic coloration in larvae of the ascidian Ecteinascidia turbinata. Mar. Biol. 96:539–544.
Young, C. M. and Chia, F.-S. 1984. Microhabitat-associated variability in survival and growth of subtidal solitary ascidians during the first 21 days after settlement. Mar. Biol. 81:61–68.
Young, C. M. and Chia, F.-S. 1987. Abundance and distribution of pelagic larvae as influences by predation, behavior, and hydrographic factors, pp. 385–463 in C. Giese and J. S. Pearse, (eds.). Reproduction in Marine Invertebrates, Vol. 9. Aberdeen University Press, Aberdeen, Scotland.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindquist, N. Chemical Defense of Early Life Stages of Benthic Marine Invertebrates. J Chem Ecol 28, 1987–2000 (2002). https://doi.org/10.1023/A:1020745810968
Issue Date:
DOI: https://doi.org/10.1023/A:1020745810968