Abstract
Purpose. The partitioning of cetirizine in a phosphatidylcholine liposomes/water system was compared with that of hydroxyzine and acrivastine to gain insight into the mechanisms of interaction of its various electrical species with membranes.
Methods. The lipophilicity profiles of the compounds were obtained from equilibrium dialysis and potentiometry, and compared with changes in NMR relaxation rates.
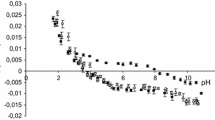
Results. The neutral form of hydroxyzine interacted mainly via hydrophobic interactions with the bilayer lipid core of the membrane, whereas for the cationic form both hydrophobic and electrostatic interactions were involved. Zwitterionic and anionic cetirizine were less lipophilic than its cation, which behaved like the corresponding species of hydroxyzine. Zwitterionic cetirizine interacted more by weak electrostatic interactions with the polar headgroups of phospholipids than by hydrophobic interactions with the membrane interior. The lipophilicity of its anion reflected the balance of repulsive electrostatic interactions between the carboxylate and phosphate groups and the hydrophobic interactions with the lipid core.
Conclusion. The study confirms that various mechanisms influence the interaction of solutes with liposomes. Combining experimental techniques and using suitable reference compounds proves useful.
Similar content being viewed by others
REFERENCES
K. K. Pandya, R. A. Bangaru, T. P. Gandhi, I. A. Modi, R. I. Modi, and B. K. Chakravarthy. High–performance thin–layer chromatography for the determination of cetirizine in human plasma and its use in pharmacokinetic studies. J. Pharm. Pharmacol. 48:510–513 (1996).
A. Pagliara, B. Testa, P. A. Carrupt, P. Jolliet, C. Morin, D. Morin, S. Urien, J. P. Tillement, and J. P. Rihoux. Molecular structure and pharmacokinetic behavior of cetirizine, a zwitterionic antihistamine. J. Med. Chem. 41:853–863 (1998).
G. Ermondi, G. Caron, G. Bouchard, G. Plemper van Balen, A. Pagliara, T. Grandi, P. A. Carrupt, R. Fruttero, and B. Testa. Molecular dynamics and NMR exploration of the property space of the zwitterionic antihistamine cetirizine. Helv. Chim. Acta (in press).
G. V. Betageri and J. A. Rogers. The liposome as a distribution model in QSAR studies. Int. J. Pharm. 46:95–102 (1988).
U. Hellwich and R. Schubert. Concentration–dependent binding of the chiral beta–blocker oxprenolol to isoelectric or negatively charged unilamellar vesicles. Biochem. Pharmacol. 49:511–517 (1995).
C. Ottiger and H. Wunderli–Allenspach. Ideal partition behaviour of acids and bases in a phosphatidylcholine liposome/buffer equilibrium dialysis system. Eur. J. Pharm. Sci. 5:223–231 (1997).
A. Avdeef, K. J. Box, J. E. A. Comer, C. Hibbert, and K. Y. Tam. pH–metric log P. 10. Determination of vesicle membrane–water partition coefficients of ionizable drugs. Pharm. Res. 15:209–215 (1998).
A. Pagliara, P. A. Carrupt, G. Caron, P. Gaillard, and B. Testa, Lipophilicity profiles of ampholytes. Chem. Rev. 97:3385–3400 (1997).
M. J. Hope, M. B. Bally, G. Webb, and P. R. Cullis. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 812:55–65 (1985).
S. D. Krämer, A. Braun, C. Jakits–Deiser, and H. W. Underli–Allenspach. Towards the predictability of drug–lipid membrane interactions: The pH–dependent affinity of propranolol to phosphatidylinositol containing liposomes. Pharm. Res. 15:739–744 (1998).
L. D. Mayer, M. J. Hope, and P. R. Cullis. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta 858:161–168 (1986).
X. R. Qi, Y. Maitani, and T. Nagai. Effect of soybean–derived sterols on the in vitro stability and the blood circulation of liposomes in mice. Int. J. Pharm. 114:33–41 (1995).
X. R. Qi, Y. Maitani, and T. Nagai. Rates of systemic degradation and reticuloendothelial system uptake of calcein in the dipalmitoylphosphatidylcholine liposomes with soybean–derived sterols in mice. Pharm. Res. 12:49–52 (1995).
K. Muramatsu, Y. Maitani, Y. Machida, and T. Nagai. Effect of soybean–derived sterol and its glucoside mixtures on the stability of dipamitoylphosphatidylcholine and dipalmitoylphosphatidylcholine/cholesterol liposomes. Int. J. Pharm. 107:1–8 (1994).
F. Frézard, C. Santaella, P. Vierling, and J. G. Riess. Permeability and stability in buffer and in human serum of fluorinated phospholipid–based liposomes. Biochim. Biophys. Acta 1192:61–70 (1994).
M. Takayama, S. Itoh, T. Nagasaki, and I. Tanimizu, A new enzymatic method for determination of serum choline–containing phospholipids. Clin. Chim. Acta 79:93–98 (1977).
P. Trinder. Determination of glucose in blood using glucose oxidase with an alernative oxygen acceptor. Ann. Clin. Biochem. 6:24–27 (1969).
S. D. Krämer, C. Jakits–Deiser, and H. Wunderli–Allenspach. Free fatty acids cause pH–dependent changes in drug–lipid membrane interactions around physiological pH. Pharm. Res. 14:827–832 (1997).
G. Caron, F. Reymond, P. A. Carrupt, H. H. Girault, and B. Testa. Combined molecular lipophilicity descriptors and their role in understanding intramolecular effects. Pharm. Sci. Technolog. Today 2:327–335 (1999).
A. Avdeef. pH–Metric log P. Part 1. Difference plots for determining ion–pair octanol–water partition coefficients of multiprotic substances. Quant. Struct.–Act. Relat. 11:510–517 (1992).
G. Caron, P. Gaillard, P. A. Carrupt, and B. Testa. Lipophilicity behavior of model and medicinal compounds containing a sulfide, sulfoxide, or sulfone moiety. Helv. Chim. Acta 80:449–462 (1997).
A. Avdeef. pH–Metric log P. II. Refinement of partition coefficients and ionization constants of multiprotic substances. J. Pharm. Sci. 82:183–190 (1993).
R. Fruttero, G. Caron, E. Fornatto, D. Boschi, G. Ermondi, A. Gasco, P. A. Carrupt, and B. Testa. Mechanisms of liposomes/water partitioning of (p–methylbenzyl)alkylamines. Pharm. Res. 15:1407–1413 (1998).
A. Kantar, J. P. Rihoux, and R. Fiorini. Effect of cetirizine on plasma membrane of human eosinophils, neutrophils and platelets: a dose response study. Eur. J. Pharm. Sci. 4:101–107 (1996).
F. E. R. Simons and K. J. Simons. Pharmacokinetic optimization of histamine H1–receptor antagonist therapy. Clin. Pharmacokin. 21:372–393 (1991).
J. P. Tillement and E. Albengres. Peut–on adapter la distribution d'un médicament dans l'organisme aux localisations de ses cibles? L'exemple d'antihistaminiques (anti H1) et de la cétirizine. Allergie et Immunologie 28:330–332 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Balen, G.P., Caron, G., Ermondi, G. et al. Lipophilicity Behaviour of the Zwitterionic Antihistamine Cetirizine in Phosphatidylcholine Liposomes/Water Systems. Pharm Res 18, 694–701 (2001). https://doi.org/10.1023/A:1011049830615
Issue Date:
DOI: https://doi.org/10.1023/A:1011049830615