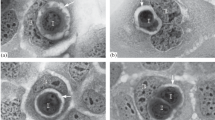
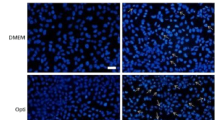
Abstract
We have addressed the possibility that Ca2+, Mg2+ and K+ ions play a central role in governing the morphological and biochemical changes attributed to apoptotic cell death. By removing Ca2+, Mg2+ or K+ ions from the cell culture medium we were able to assess the contribution of each ion to hybridoma cell growth and viability. The differences were explained in terms of a possible reduction in their respective intracellular levels. From several lines of evidence, the deprivation of K+ ions was the most detrimental to cellular growth and viability and induced significant levels of early apoptotic cells. Another effect of this deprivation was to weaken the plasma membranes without causing membrane breakdown; exposure to high agitation rates confirmed fragility of the cell membranes. Removal of Mg2+ caused a reduction in the levels of early apoptotic cells and predisposed cells to high levels of primary necrotic death. The lower levels of apoptotic cells failed to demonstrate the classic nuclear morphology associated with apoptosis, while retaining other apoptotic features. These results highlighted the importance of utilizing several assays for the determination of apoptosis. The absence of Ca2+ appeared to be the mildest insult, but its deprivation did accelerate a significant decline in culture by increasing apoptotic death. Hybridoma cells overexpressing the apoptotic suppresser gene bcl-2 were protected from the predominantly necrosis inducing effects of Mg2+ ion deprivation and apoptosis inducing effects of Ca2+ ion deprivation. However, apoptosis was not as effectively suppressed in bcl-2 cells responding to incubation in K+ free medium. The inclusion of bcl-2 activity in the mechanisms of Ca2+ Mg2+ or K+ deprivation induced cell death emphasizes a close relationship between ionic dissipation and the apoptotic process.
Similar content being viewed by others
References
Wyllie AH, Beattie GJ, Hargreaves AD. Chromatin changes in apoptosis. Histochem J 1981; 13: 681.
Nicotera P, Zhivitovsky B, Orrenius S. Nuclear calcium transport and the role of calcium in apoptosis. Cell Calcium 1994; 16: 279–288.
McConkey DJ, Hartzell P, Nicotera P, Orrenius S. Calcium activated DNA fragmentation kills immature thymocytes. Faseb J 1989; 3: 1839–1849.
McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Biochem Biophys 1989; 269: 365–370.
Duke RC, Witter RZ, Nash PB, Young JDE, Ojcius DM. Cytolysis mediated by ionophores and pore-forming agents: role of intracellular calcium in apoptosis. Faseb J 1994; 8: 237–246.
Cotter TG, Fernanded RS. Activation of a calcium magnesium independent endonuclease in human leukemic cell apoptosis. Anticancer Res 1993; 13: 1253–1259.
Orrenius S, McCabe MJ, Nicotera P. Ca2+-dependent mechanisms of cytotoxicity and programmed cell death. Toxicol Lett 1992; 64/65: 357–364.
Walker PR, Sikorska M. New aspects of the mechanism of DNA fragmentation in apoptosis. Biochem Cell Biol 1997; 75: 287–299.
Cain K, Salmaan H, Hussain I, Kokileva L, Cohen GM. DNA cleavage in rat liver nuclei activated by Mg2+ or Ca2+ + Mg is inhibited by a variety of structurally unrelated inhibitors. Biochem Cell Biol 1994; 72: 631–638.
McCarthy JV, Cotter TG. Cell shrinkage and apoptosis: a role for potassium and sodium ion efflux. Cell Death and Differentiation 1997; 4: 756–770.
Dallaporta B, Hirsch T, Susin SA, et al. Potassium leakage during the apoptotic degradation phase. J Immunol 1998; 160: 5605–5615.
Bortner CD, Huges FM, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem 1997; 272: 32436.
Hoffman EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cell. Physiol Rev 1989; 69: 315–382.
Muchmore SW, Sattler M, Liang H, et al. X-ray and NMR structure of human Bcl- XL , an inhibitor of programmed cell death. Nature 1996; 381: 335.
Minn AJ, Vélez P, Schendel SL, et al. Bcl-XL forms ion an ion channel in synthetic lipid membranes. Nature 1997; 385: 353–357.
Singh RP, Al-Rubeai M, Gregory CD, Emery AN. Cell death in bioreactors: a role for apoptosis. Biotechnol Bioeng 1994; 44: 720.
Al-Rubeai M, Emery AN, Chalder S. Flow cytometric study of cultured mammalian cells. J Biotechnol 1991; 19: 67.
Ishaque A, Al-Rubeai M. Use of intracellular pH and annexin-V flow cytometric assays to monitor apoptosis and its suppression by bcl-2 over-expression in hybridoma cell culture. J Immunol Methods 1998; 221: 43–57.
Welsh JP, Al-Rubeai M. The Relationship between Intracellular pH and Cell Cycle in Cultured Animal Cells using SNARF-1 Indicator. Flow Cytometry Applications in Culture, In: Al-Rubeai M and Emery AN, eds., Marcel Dekker, 1996: 163–175.
Hampton MB, Vanags DM, Pörn-Ares I, Orrenius S. Involvement of extracellular calcium in phoshatidylserine exposure during apoptosis. FEBS Lett 1996; 399: 277–282.
Flatman PW. The control of red cell magnesium. Magnesium Res 1988; 1: 5–11.
Sambrook JF. The involvement of calcium transport secretory proteins from the endoplasmic reticulum. Cell 1990; 61: 197–199.
Bellomo G, Perotti M, Taddei F, et al. Tumor necrosis factor induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear Ca2+ concentration and DANN fragmentation. Cancer Res 1992; 52: 1342–1346.
Ojcius DM, Zynchinsky A, Zheng LM, Young JDE. Ionophore-induced apoptosis: Role of DNA fragmentation and calcium fluxes. Exp Cell Res 1991; 197: 43–49.
Gottlieb RA. Cell acidification in apoptosis. Apoptosis 1996; 1: 40.
Reynolds JE, Li J, Craig RW, Eastman A. Bcl-2 and MCL-1 expression in Chinese hamster ovary cells inhibits intracellular acidification induced by staurosporine. Exp Cell Res 1996; 225: 430.
Meisenholder GW, Martin SJ, Green DR, Nordberg J, Bernard MB, Gottlieb RA. Events in apoptosis: acidifiaction is downstream of protease activation and bcl-2 protection. J Biol Chem 1996; 271: 16260.
He H, Lam HH, McCormick TS, Distelhorst CW. Maintenance of calcium homeostasis in the endoplasmic reticulum by bcl-2. J Cell Biol 1998; 138:19976; 1219–1228.
Martin SJ, Reutelingsperger CPM, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of bcl-2 and abl. J Exp Med 1995; 182: 1545–1556.
Bowmen ID, Bowmen SM. Programmed Cell Death in Tumours and Tissues. London: Chapman and Hall, 1990.
Evans GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992; 69: 119–128.
Vairo G, Inmes KM, Adams JM. Bcl-2 has a cell cycle inhibitory function separable from its enhancement of cell survival. Oncogene 1996; 13: 1511–1519.
Borner C. Diminished cell proliferation associated with the death protective activity of bcl-2. J Biol Chem 1996; 271: 12695–12698.
Simpson NH, Singh RP, Emery AN, Al-Rubeai. Bcl-2 overexpression reduces the growth rate and prolongs G1 phase in continuous chemostat cultures of hybridoma cells. Biotechnol Bioeng 1999; 64: 174–186.
Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia induced cell death by bcl-2 and bcl-x L . Nature 1995; 374: 811–813.
Shimizu S, Eguchi Y, Kamiike W, et al. Retardation of chemical hypoxia-induced necrotic cell death by bcl-2 and ICE inhibitors possible involvement of common mediators in apoptotic and necrotic signal transduction: Oncogene 1996; 12: 2045–2050.
Hirsch T, Marchetti P, Susin SA, et al. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene 1997; 15: 1573–1581.
Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of bcl-2 like oncoproteins. Oncogene 1998; 16: 2265–2282.
Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res 1998; 51: 293–308.
Yu SP, Yeh CH, Sensi SL, et al. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 1997; 278: 114.
D'mello SR, Galli C, Ciotti T, Calissano. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of cell death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA 1993; 90: 10989.
Marakhova II, Vereninov AA, Toropova FV, Vinogrdova TA. Na+, K+-ATPase pump in activated human lymphocytes: on the mechanisms of rapid and long-term increase in K+ influxes during the initiation of phytohemagglutinin-induced proliferation. Biochimica et Biophysica Acta 1998; 1368: 61–72.
Al-Rubeai M, Emery AN, Chalder S, Goldman MH. A flow cytometric study of hydrodynamic damage to mammalian cells. J Biotechnol 1993; 31: 161–177.
Al-Rubeai M, Singh RP, Goldman MH, Emery AN. Death mechanisms of animal cells in conditions of agitation. Biotechnol Bioeng 1995; 45: 463.
Que FG, Gores GJ, Larusso NF. 1. Development and initial application of an in vitro model of apoptosis in rodent cholangiocytes. Am J Phsiol 1997; 272: (Gastrointest Liver Physiol 35): G106-G115.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ishaque, A., Al-Rubeai, M. Role of Ca2+, Mg2+ and K+ ions in determining apoptosis and extent of suppression afforded by bcl-2 during hybridoma cell culture. Apoptosis 4, 335–355 (1999). https://doi.org/10.1023/A:1009643204200
Issue Date:
DOI: https://doi.org/10.1023/A:1009643204200