Abstract
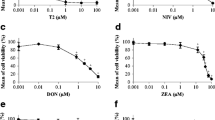
Deoxynivalenol (DON) is a trichothecene mycotoxin produced by various species of fungi. Trichothecenes are known as major contaminants of cereals and cereal-containing foods. DON has been detected in agricultural products worldwide and persists in products after processing. In humans as well as in animals, DON has been shown to induce both alimentary and hematological toxicities. Granulo-monocytic progenitors (CFU-GM) from human umbilical cord blood from rat bone marrow were cultured in the presence of DON (from 10-6 to 10-8 mol/L) for 14 days. DON rapidly inhibits human and rat CFU-GM in a concentration-dependent manner between 10-6 and 2.5 × 10-7 mol/L. IC50 values on days 7, 10, and 14 were, respectively, 3 × 10-8, 2.9 × 10-8, 3.9 × 10-8 mol/L for human CFU-GM and 2.6 × 10-7, 1.5 × 10-7, and 1.6 × 10-7 mol/L for rat CFU-GM. The present study defines the cytotoxic and inhibitory DON concentrations for rat and human CFU-GM and provides a system for further investigation of cellular DON targets and elucidation of the mechanism of trichothecene hematotoxicity. Moreover, we propose one of the trichothecenes tested in our studies as a reference molecule for in vitro studies, since one mycotoxin seems to be the most potent myelotoxic inhibitor of CFU-GM detected to date.
Similar content being viewed by others
References
Abbas HK, Mirocha CJ, Pawlosky RJ, Pusch DJ. Effect of cleaning, milling, and baking on deoxynivalenol in wheat. Appl Environ Microbiol. 1985;50:482–6.
Atkinson HAC, Miller K. Inhibitory effect of deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction of rat and human lymphocyte proliferation. Toxicol Lett. 1984;23: 215–21.
Ayral AM, Dubech N, Le Bars J, Escoula L. In vitro effect of diacetoxyscirpenol and deoxynivalenol on microbicidal ac-tivity of murine peritoneal macrophages. Mycopathologia. 1992;120:121–7.
Boorman GA, Luster MI, Dean JH, Campbell ML. Assess-ment of myelotoxicity caused by environmental chemicals. Environ Health Perspect. 1982;43:129–35.
Ciegler A. Trichothecenes: occurrence and toxicoses. J Food Protect. 1978;41:399–403.
Cisti A, Faure J, Faugere JG, Narbonne JF. Les toxines trichothécènes-aspects généraux et toxicologiques. Cah Nutr Diét. 1983;18:317–23.
Cronkite EP. Kinetics of granulocytopoiesis. Clin Haematol. 1979;8:351–70.
Deldar A, House RV, Wiera D. Bone marrow colony-forming assays. Methods Immunotoxicol. 1995;1:227–50.
Fernandez C, Stack ME, Musser SM. Determination of deoxy-nivalenol in 1991 U.S. winter and spring wheat by high-performance thin-layer chromatography. J Assoc Off Anal Chem. 1994;77:628–30.
Forsyth DM, Yoshizawa T, Morooka N, Tuite J. Emetic and refusal activity of deoxynivalenol to swine. Appl Environ Microbiol. 1977;34:547–52.
Gribaldo L, Bueren J, Deldar A et al. Evaluation of haemato-toxic effects using in vitro systems. The report and recom-mendations of ECVAM Workshop 17. ATLA. 1996;24:211–31.
Hadidane R, Roger-Regnault C, Bouattour F, Bacha H, Creppy EE, Dirheimer G. Correlation between alimentary myco-toxin contamination and specific diseases. Hum Toxicol. 1985;4:491–501.
Lautraite S, Parent-Massin D, Rio B, Hoellinger H. Compar-ison of toxicity induced by T-2 toxin on human and rat granulo-monocytic progenitors with an in vitro model. Hum Exp Toxicol. 1995;14:672–8.
Lautraite S, Parent-Massin D, Rio B, Hoellinger H. Compar-ison of toxicity by HT-2 toxin on human and rat granulo-monocytic progenitors with an in vitro model. Hum Exp Toxicol. 1996;15:208–13.
Leglise C, Tarode de Tailly P, Vignot JL, Le Bot MA, Le Roux AM, Riché C. A cellular model for drug interactions on hematopoiesis: the use of human umbilical cord blood as a model for the study of drug-related myelosuppression of normal hematopoiesis. Cell Biol Toxicol. 1996;12:39–53.
Metcalf D. Clonal culture of hemopoietic cells: techniques and applications. Amsterdam: Elsevier Science; 1984:1–167.
Mirocha CJ, Pawlosky RJ, Chatterjee K, Watson S, Hayes W. Analysis for Fusarium toxins in various samples implicated in biological warfare in southeast Asia. J Assoc Off Anal Chem. 1983;66:1485–99.
Naughton BA, Sibanda D, Azar L, San Roman J. Differential effects of drugs upon hematopoiesis can be assessed in long-term bone marrow cultures established on nylon screens. Proc Soc Exp Biol Med. 1992;199:481–90.
Noble C, Sina JF. Usefulness of the in vitro bone marrow colony-forming assay in cellular toxicology. In Vitro Tox-icol. 1993;6:187–95.
Parchment RE, Huang M, Erikson-Miller CL. Roles for in vitro myelotoxicity tests in preclinical drug development and clinical trial planning. Toxicol Pathol. 1993;21:241–50.
Parent-Massin D, Thouvenot D. In vitro study of pesticide hematotoxicity in human and rat progenitors. J Pharmacol Toxicol Methods. 1993;30:203–7.
Parent-Massin D, Deslandes E, Hourmant N, Ledain A, Thouvenot D, Riché C. Toxicological evaluation of semi-refined carrageenans by two models of cell culture: hepato-cytes and hematopoietic progenitors from rat. Sci Aliments. 1993;13:305–10.
Parent-Massin D, Fuselier R, Thouvenot D. In vitro toxicity of trichothecenes on human hemaotpoietic progenitors. Food Addit Contam. 1994a;11:441–7.
Parent-Massin D, Thouvenot D, Rio B, Riché C. Lindane haematotoxicity confirmed by in vitro tests on human and rat progenitors. Hum Exp Toxicol. 1994b;13:103–6.
Parent-Massin D, Thouvenot D. In vitro toxicity of trichothe-cenes on rat haematopoietic progenitors. Food Addit Con-tam. 1995;12:41–9.
Pestka JJ, Tai J-H, Witt MF, Dixon DE, Forsell JH. Suppres-sion of immune response in the B6C3F1 mouse after dietary exposure to the Fusarium mycotoxins deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 1987;25: 297–304.
Rabie CJ, Sydenham EW, Thiel PG, Lübben A, Marasas WFO. T-2 toxin production by Fusarium acuminatum isolated from oats and barley. Appl Environ Microbiol. 1986;52:594–6.
Rizzo AF, Atroshi F, Hirvi T, Saloniemi H. The hemolytic activity of deoxynivalenol and T-2 toxin. Natural Toxins. 1992;1:106–10.
Robbana-Barnat S, Loridon-Rosa B, Cohen H, Lafarge-Frayssinet C, Neish GA, Frayssinet C. Protein synthesis inhibition and cardiac lesions associated with deoxynival-enol ingestion in mice. Food Addit Contam. 1987;4:49–55.
Robbana-Barnat S, Lafarge-Frayssinet C, Cohen H, Neish GA, Frayssinet C. Immunosuppressive properties of deoxy-nivalenol. Toxicology. 1988;48:155–66.
Schlunck T, Schleyer M. The influence of culture conditions on the production of colony stimulating activity by human placenta. Exp Hematol. 1980;8:179–84.
Scott PM, Kanhere SR, Dexter JE, Brennan PW, Trenholm HL. Distribution of the trichothecene mycotoxin deoxynivalenol (vomitoxin) during the milling of naturally contaminated hard red spring wheat and its fate in baked products. Food Addit Contam. 1984;1:313–23.
Tanaka T, Hasegawa A, Yamamoto S, Lee U-S, Sugiura Y, Ueno Y. Worldwide contamination of cereals by the Fusar-ium mycotoxins nivalenol, deoxynivalenol, and zearalenone. 1. Survey of 19 countries. J Agric Food Chem.} 1988};36}:979–
Tanaka T, Ueno Y. Worldwide natural occurrence of Fusarium mycotoxins, nivalenol, deoxynivalenol and zearalenone. In: Natori S, Hashimoto K, Ueno Y, eds. Mycotoxin and phycotoxins '88. Amsterdam: Elsevier Science; 1989:51–6.
Tebbi K, Rubin S, Cowan DH, McCulloch EA. A comparison of granulopoiesis in culture from blood and marrow cells of non-leukemic individuals and patients with acute leukemia. Blood. 1976;48:235–43.
Ueno Y. Trichothecenes-chemical, biological, and toxicologi-cal aspects. 4. Amsterdam, Elsevier Science; 1983:1–313.
Vesonder RF, Ciegler A, Jensen AH. Isolation of the emetic principle from Fusarium-infected corn. Appl Microbiol. 1973;26:1008–10.
Warner RL, Brooks K, Pestka JJ. In vitro effects of vomitoxin (deoxynivalenol) on T-cell interleukin production and IgA secretion. Food Chem Toxicol. 1994;32:617–25.
World Health Organization. Environmental health criteria for selected mycotoxins: ochratoxins, trichothecenes, and ergot. Geneva: WHO Publications; 1996:1–263.
Yoshizawa T, Morooka N. Studies on the toxic substances in the infected cereals. III. Acute toxicities of new trichothe-cene mycotoxins: deoxynivalenol and its monoacetate. J Food Hyg Soc Jpn. 1974;15:261–5.
Zakharova LP, Obol'skii OL, L'vova LS, Kravchenko LV. Deoxynivalenol cereal contamination in Russia. Voprosy Pitaniya O. 1994;3:40–4.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lautraite, S., Parent-Massin, D., Rio, B. et al. In vitro toxicity induced by deoxynivalenol (DON) on human and rat granulomonocytic progenitors. Cell Biol Toxicol 13, 175–183 (1997). https://doi.org/10.1023/A:1007306212898
Issue Date:
DOI: https://doi.org/10.1023/A:1007306212898