Abstract
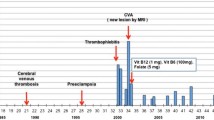
Among 40 patients with homocystinuria due to cystathionine β-synthase deficiency diagnosed in the state of New South Wales, Australia (population 6 million) and followed long-term, there were 10 deaths at ages 2-30 years. Of these 8 were definite vascular deaths, one was a presumed vascular death, and the other was due to an accident and unrelated to homocystinuria. The vascular deaths were all early cases and only one patient, a pyridoxine-responsive 30-year-old woman, had been prescribed adequate treatment although it was uncertain that she was taking it.
In 32 patients of mean age 30 years (range 9-66 years) there were 539 patient-years of treatment with pyridoxine, folic acid and hydroxocobalamin. There were 17 pyridoxine-responsive patients and all maintained plasma total free homocyst(e)ine levels <200µmol/L over an average treatment period of 16.6 years. The 15 non-responsive patients received additionally 6-9 g of betaine daily. This resulted in a further 74% mean decline (±14% SD) in plasma total free homocyst(e)ine, persisting during an average (post-betaine) treatment period of 11 years; current mean ± SD levels are 33 ± 17 µmol/L (n=15).
There were two vascular events during treatment, one fatal pulmonary embolus (see above) and one myocardial infarction, whereas without treatment, 21 would have been expected, χ2 = 14.22, p = 0.0001, relative risk 0.09 (95% CI 0.02-0.38). There were no events during 258 patient-years of treatment in the 15 pyridoxine-nonresponsive patients (p<0.005 versus expected untreated). Nineteen patients had a total of 19 major and 15 minor operations requiring anaesthetic, and three had successful pregnancies, one whilst receiving betaine. There were no thromboembolic complications.
We conclude that treatment which effectively lowers circulating homocyst(e)ine, even to suboptimal levels, markedly reduces cardiovascular risk in patients with cystathionine β-synthase deficiency, and that betaine therapy contributes importantly to this in pyridoxine-nonresponsive patients. Betaine as additional therapy is safe and effective for at least 16 years.
Similar content being viewed by others
REFERENCES
Bendich A, Cohen M (1990) Vitamin B6 safety issues. Ann NY Acad Sci 585: 321–330.
Celemajer DS, Sorensen K, Ryalls M, et al (1993) Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol 22: 854–858.
Mandel H, Brenner B, Berant M, et al (1996) Coexistence of hereditary homocystinuria and factor V Leiden — effect on thrombosis. New Engl J Med 334: 763–768.
Mayer EL, Jacobsen DW, Robinson K (1996) Homocysteine and coronary atherosclerosis. J Am Coll Cardiol 27: 517–527.
Motulsky AG (1996) Nutritional ecogenetics: homocysteine-related arteriosclerotic vascular disease, neural tube defects, and folic acid. Am J Hum Genet 58: 17–20.
Mudd SH, Skovby F, Levy HL, et al (1985) The natural history of homocystinuria due to cystathionine β-synthase deficiency. Am J Hum Genet 37: 1–31.
Mudd SH, Levy HL, Skovby F (1995) Disorders of transsulfuration. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease, 7th edn. New York: McGraw-Hill, 1279–1327.
Smolin LA, Benevenga J, Berlow S (1981) The use of betaine for the treatment of homocystinuria. J Pediatr 99: 467–472.
Stamler JS, Osborne JA, Jaraki O, et al (1993) Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest 91: 308–318.
Tsai JC, Wang H, Perrella MA, et al (1996) Induction of cyclin A gene expression by homocysteine in vascular smooth muscle cells. J Clin Invest 97: 146–153.
Wilcken DEL, Dudman NPB (1992) Homocystinuria and atherosclerosis. In Lusis AJ, Rotter JI, Sparkes RS, eds. Molecular Genetics of Coronary Artery Disease. Candidate Genes and Processes in Atherosclerosis. Monogr Hum Genet, vol. 14. Basel: Karger, 311–324.
Wilcken B, Turner B (1973) Homocystinuria: reduced folate levels during pyridoxine treatment. Arch Dis Child 48: 58–62.
Wilcken DEL, Wilcken B (1976) The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J Clin Invest 57: 1079–1082.
Wilcken DEL, Wilcken B, Dudman NPB, Tyrrell PA (1983) Homocystinuria: the effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med 309: 448–453.
Wiley V, Dudman NPB, Wilcken DEL (1988) Interrelations between free and bound homocysteine and cysteine in homocystinuria. Metabolism 37: 191–195.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilcken, D.E.L., Wilcken, B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inherit Metab Dis 20, 295–300 (1997). https://doi.org/10.1023/A:1005373209964
Issue Date:
DOI: https://doi.org/10.1023/A:1005373209964