Abstract
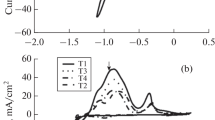
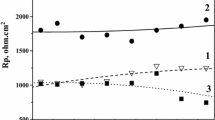
The aqueous corrosion resistances of Zn and Zn–Sn (∼ 20 wt% Sn) electrodeposits, passivated by immersion in chromating solution with different ratios of Cr(vi) to activating ions, are compared. The electrochemical behaviour of various chromated and nonchromated coatings were investigated in deaerated 0.5 mol dm−3 Na2SO4/pH 5 solution using a.c. impedance and d.c. polarization techniques. The polarization curves revealed that the chromate layers influence both the cathodic and anodic reactions. The corrosion rate of each specimen decreases with time due to the accumulation of corrosion products. The dark yellow (DY) chromate film on the Zn–Sn alloy and the iridiscent yellow (IY) on Zn yields the best protective ability in agreement with the assessment of prolonged salt spray chamber tests. These chromate layers resembling cracked mud become permeable to the electrolyte after immersion and, as a consequence of the transformation and the leaching of certain Cr compounds, a very porous agglomerate of corrosion products forms. The morphology and structure of dark yellow chromated Zn–Sn alloy was also investigated by transmission electromicroscopy (TEM) and scanning electronmicroscopy with microprobe (SEM/EDS) analyses before and after corrosion. The depth profile of the corroded surface chemical composition was determined by X-ray photoelectron spectroscopy (XPS).
Similar content being viewed by others
References
L. Sziráki, H. Csontos, M.L. Varsányi and L. Kiss, Corros. Sci. 35 (1993) 371.
A. Brenner, Electrodeposition of Alloys, Academic Press, New York, vol. 2 (1963) p. 53.
G.D. Wilcox and D.R. Gabe, Corros. Sci. 35 (1993) 1251.
G. Bech-Nielsen and G.D. Juhl, Corros. Sci. 34 (1993) 785.
E. Budman, Proc. AESF Aerospace Symp., Orlando, FL (29–30 Jan. 1992), p. 129.
St. Vitkova, V. Ivanova and G. Raichevsky, Surf. Coat. Technol. 82 (1996) 226.
V. Ivanova, G. Raichevsky, St. Vitkova and M. Nikolova, Surf. Coat. Technol. 82 (1996) 232.
G. Raichevsky, V. Ivanova, St. Vitkova and M. Nikolova, Surf. Coat. Technol. 82 (1996) 239.
J. Dévai and L. Mészáros, Acta Chim. Acad. Hung. 100 (1979) 183; L. Mészáros, G. Mészáros and B. Lengyel, J. Electrochem. Soc. 141 (1994) 2068.
L. Weng, G. Vereecke, M. Genet, P. Berrand and W. Stone, Surf. Inter. Anal. 20 (1993) 179.
J. Scofield, J. Electron Spectrosc. Relat Phenom. 8 (1972) 12.
L. Sziráki and L. Kiss, ACH-Models in Chemistry 131 (1994) 581.
V.I. Nefedov, Rentgenoelektroskopija Chemicheskich Soedinenija, Chimija, Moskow (1984).
G.E. Muilenberg (ed.), Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corporation, Physical Electronics Division, MN (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SZIRÁKI, L., CZIRÁKI, Á., VÉRTESY, Z. et al. Zn and Zn–Sn alloy coatings with and without chromate layers. Part I: Corrosion resistance and structural analysis. Journal of Applied Electrochemistry 29, 927–937 (1999). https://doi.org/10.1023/A:1003584420237
Issue Date:
DOI: https://doi.org/10.1023/A:1003584420237