Abstract
Microbial induced carbonate precipitation (MICP) is relatively an innovative soil improvement technique, learnt from the bio-mediated geochemical reactions that naturally occur in the earth surface. During the MICP, CaCO3 is metabolically precipitated in soil pores, cement the particle contacts and improves the strength and stiffness of soil. Environment temperature is one of the most key factors that determines the efficiency MICP. The purpose of this study is to investigate the feasibility of stabilizing the slope soil of cold subarctic region (Hokkaido, Japan). The implication of MICP in cold subarctic zones remains as a major challenge, as the enzymatic performance of the bacteria typically declines during lower temperatures hence insufficient formation of CaCO3 in soil matrix. Therefore, as a potential approach, this study attempted to investigate the feasibility of using the bacteria which have been adapted to native cold climatic conditions. The objectives of this paper are evaluating (1) the effect of temperature in bacterial response, and (2) the effect of grain size distribution in cementation mechanism. The observations suggest that the enzyme activity of the bacteria is negligible at and above 30 °C, whereas it is significant at relatively lower temperatures. The comparison of treated soils suggests that the fine content in slope soil increased number of particle contacts, facilitated effective packing, and promoted the effectiveness of MICP compared to that of uniformly graded sands. Finally, the technical feasibility in slope soil stabilization was well demonstrated using model solidification test. The limitations in stabilizing the slope are also discussed in detail.
Similar content being viewed by others
1 Introduction
MICP is an innovative biological ground improvement technique that could be applicable to many geo-engineering problems. The process utilizes the ureolytic bacteria to induce bio-cementation in a sustainable and cost-effective manner. The urease enzyme produced by the bacteria catalyses the hydrolysis of urea into ammonium and carbonic acid (Eq. 1), hence increases the alkalinity of the reaction medium. In the presence of calcium ions, the calcium carbonate cement is precipitated at the nucleation sites provided by the bacteria cells (Eq. 2) [1,2,3].
The precipitated carbonate crystals coat the soil particles, cement the soil matrix, fill the voids partially, hence increases the desired mechanical properties including strength and stiffness of the soil matrix [4, 5]. Despite being a relatively new technique, many MICP studies have been investigated for several geotechnical applications including liquefaction control, erosion mitigation, coastline preservation, sand dunes stabilization, borehole stabilization and settlement control [6,7,8,9]. Most of the investigations have been focused on the behavior of MICP treated sand, providing information on the strength and stiffness characteristics under different testing conditions [2, 5, 6, 9,10,11]. The studies to promote MICP applications need to be expanded, for soil types other than sand materials. Few researchers including van Paassen [12] and Mortensen et al. [13] have attempted the investigation on gravel and different soils ranging from very fine to very coarse and suggested that large amount of carbonate precipitation would be required to strengthen the coarse-gravel material. Also, the free passage of microorganisms through the limited pore-throat size remains a challenge in treating the very fine soils using MICP [14]. Recently, researchers have started to focus on assessing the performance of MICP in residual soils that consist fine to coarse grains distributed naturally [14,15,16]. However, only a very limited MICP studies on residual soils have been reported in the literature to the date, providing insufficient information on their mechanical responses.
The MICP treatment can be achieved by stimulating the growth of indigenous bacteria in situ (bio-stimulation) or by augmenting ureolytic bacterial culture (bio-augmentation). Bio-stimulation is a process of modifying the in situ environmental conditions to enrich the existing microbial community with required urease capabilities [17, 18]. Bio-augmentation largely differs from bio-stimulation and can be enabled by two ways: (1) bio-augmentation of indigenous bacteria: injecting enriched culture of indigenous bacteria isolated from native soil, and (2) bio-augmentation using exogenous bacteria: injecting enriched culture of specialized non-native bacterial strains (e.g. Sporosarcina pasteurii). Bio-augmentation by exogenous bacteria has been extensively researched and demonstrated at a variety of laboratory scales [1, 5, 6, 11, 13], whereas the studies concerned on bio-augmentation using indigenous bacteria up to now are very limited [9, 19].
The augmentation of indigenous bacteria offers important environmental benefit through the elimination of nonnative bacterial supply (microbial pollution) into natural soil ecosystems [8]. Although the native species perform the urea hydrolysis at slower rates compared to that of specialized strains (e.g. Sporosarcina pasteurii), they would be more reliable than the exogenous strains in their native environment, resulting advantages in MICP with respect to the fitness of the strains and sustained enzymatic capabilities. At the same time, introducing non-native bacteria to a new environment may hinder the survival and enzymatic performance of the strains [20, 21], and the process may also face some ambiguities with respect to regulatory acceptance [21].
The selection of suitable ureolytic bacteria is highly important in urease-based MICP technique. The enzymes of the bacteria are thermal sensitive and can readily be denatured by even a slight change of environmental temperature [22]. Most of the MICP studies have employed Sporosarcina pasteurrii for urea hydrolysis due to the highly active urease enzyme [5, 6, 11, 13, 23,24,25]. Only a few MICP studies focused on introducing some other ureolytic strains including Bacillus sphaercus (MCP-11) [26], Pararhodobactor sp. [9, 27], Bacillus sp. VS1 [28, 29] and B. megaterium (ATCC 14581) [14]. However, most of the soil bacteria reported to date could effectively produce urease enzyme only within moderate to high temperature ranges (25 °C–60 °C), and within the range, the activity increased with increase in temperature [14, 30]. It must be noted that the functional temperature range of the enzymatic reaction is required to cover the temperature of the field stratum to be treated for an effective carbonate precipitation. Since the performance of the bacteria becomes negligible at low temperatures, implications of MICP in cold climatic zones remain as a huge challenge.
The study reported herein presents an initial level investigation on stabilization of a residual soil from the slope located in cold subarctic zone (Hokkaido, Japan). The novel indigenous ureolytic bacteria which had been adapted to the cold native environmental conditions were isolated from the native slope soil and investigated in detail. Effect of CaCO3 precipitation on the process of strengthening in slope soil was compared with three uniformly graded soils with distinct mean particle size. The morphology and the spatial distribution of CaCO3 were analysed at the microscale using scanning electron microscopy (SEM). The results obtained from this study would be beneficial to the future work that is to introduce the MICP technique as a novel candidate for surface stabilization of the slope.
2 Materials and method
2.1 Soil characteristics
The grain size distribution curves of the soils studied in this work are given in Fig. 1. Mizunami, Mikawa and Toyoura sands are standard laboratory sands, uniformly graded: SP based on Unified Soil Classification System (USCS) [31] with mean particle sizes (D50) of 1.6 mm, 0.87 mm and 0.2 mm respectively. The slope soil considered in the current study is from expressway slope of Onuma, Hokkaido, Japan (Latitude 42.388532 and Longitude 140.284762). It is a well graded sand (SW based on USCS, D50 = 0.23 mm), consisting very fine sand content (grain size < 125 μm) of 12%. pH value of the slope soil is 6.997 which is very close to that of uniformly graded soils (7.0 ± 0.1). Similar to the standard sands, the organic content in slope soil is found to be negligible. A summary of the X-Ray diffraction results of all the soils considered herein is presented in Table 1. The results showed that the silica is the dominant mineral in all the soils.
2.2 Isolation and characterization of indigenous bacteria
Isolation and characterization of bacteria was performed in accordance with the method suggested in previous studies [9, 32]. Soil sample collected from the study location (in sterile test tubes) was employed in laboratory for identifying bacteria. 5 g of soil sample was diluted to 101–105 in separate sterile test tubes with autoclaved distilled water. The solutions were applied on NH4-YE agar plates (prepared by combining tris-buffer, ammonium sulfate, yeast extract and ager), followed by the incubation at 30 °C for 72 h. Formed colonies were then isolated and grown on separate new NH4-YE agar plates. Each colony was employed in cresol-red test by transferring the colony into 20 mL cresol-red test solution (combined of cresol-red, urea, ethanol and distilled water), followed by the incubation at 45 °C for 2 h. The ureolytic bacteria were identified based on the pH (due to the formation of ammonium ions by hydrolyzing the urea) and color change (from yellow to purple) of the solution. The identified ureolytic strains were characterized by sequencing their 16S rDNA and comparing the results to sequences available in the Apollon DB-BA 9.0 database, GenBank, DDBJ (DNA Data Bank of Japan) and EMBL (European Molecular Biology Laboratory).
2.3 Reactant characteristics
The bacteria culture was enriched in sterile NH4-YE medium consisting tris-buffer (15.7 g/L), ammonium sulfate (10 g/L) and yeast extract (20 g/L) with a neutral pH value, aerobically in a shaking incubator at 160 rpm. The cementation reagent (1 mol/L) for the MICP treatment consisted of calcium chloride (111 g/L), urea (60 g/L) and nutrient broth (6 g/L).
2.4 Urease activity measurements
Urease activity of the bacteria was measured using spectrophotometric determination of ammonia as Indophenol [33]. 1 mL of bacterial culture was added to the 0.1 mol/L urea prepared in phosphate buffer solution. The ammonium ions produced from the urea hydrolyses could react with phenol at the presence of hypochlorite and produce the blue colour indophenol dye in the alkaline medium. The intensity of indophenol dye was measured at the wave length of 630 nm (OD630) at different uniform intervals of catalysation (at every 5 min interval). Using the calibration curve developed between concentration of ammonium ions and intensity (OD630), the rates of urea hydrolysis were estimated.
2.5 Soil column test
Soil columns (6 cm in height, 2.5 cm diameter) were prepared using 35 mL standard syringes and positioned vertically (Fig. 2). The columns of all the soils were packed to a dry density of 1.6 ± 0.1 g/cm3 similar to that reported by Cheng and Cord-Ruwisch [3]. Reactants were injected in two subsequent phases as suggested by several MICP researchers [1, 2, 11]. In the first phase, the enriched bacteria culture was injected to the column followed by a time gap of 2 h to immobilize the bacteria cells with soil particles. In the second phase, cementation solution was injected at a constant flow rate of 2–4 mL/min. All the solutions were simply applied to the top surface of the soil columns and allowed to percolate under gravitational and capillary forces. During the treatment, the columns were percolated with cementation solution every 24 h (total of 14 cementation injections, 10 mL per each), whereas the columns were filled by bacteria culture once again at the middle of the fourteen days treatment period (total of 2 biological injections, 10 mL per each). The pH and Ca2+ concentration of the drainage were measured every 72 h. After the treatment was completed, the samples were flushed with distilled water prior to the strength measurements.
2.6 Slope model test
The slope model (13 × 10 × 10 cm) was filled with slope soil by five layers, and each layer was compacted evenly to a similar dry density used in column test. The gradient of the slope was resolved to the standard gradient of expressway cut slope in accordance with the road earthwork guidelines of Japan [34, 35]. During the treatment, a grid-based injection method was performed. The slope surface was divided into 8 × 5 grids, each grid was 2 cm × 2 cm, and the solutions were injected on the surface of the slope and allowed to percolate under gravitational forces. Based on the pore volume of the soil below each grid, the injection volumes were estimated, and the rate of injection was 8–10 mL/min. Similar to the column test, 2 number of biological injections and 14 number of cementation injections were performed totally during 14 days of treatment process. The experimental arrangement of slope model test is illustrated in Fig. 3.
2.7 Uniaxial compressive strength (UCS) measurement
The UCS of the cemented columns and slope were estimated using needle penetrometer (SH-70, Maruto Testing Machine Company, Tokyo, Japan) according to JGS 3431-2012 [36]. The equipment was developed in Japan for predicting the UCS of soft, weak, very weak rocks and cemented soil specimens, and recently, ISRM has recommended the method for indirect estimation of mechanical properties of the soft rock materials [37]. This penetration is a non-destructive test, and that has a great potential to be used as a sound technique for MICP field assessments. The penetration depth (mm) of the needle attached to the device and the penetration resistance (N) were measured during the test. Using the regression relationship (Eq. 3) developed by analyzing 114 natural soft rock samples and 50 cemented soil samples, the UCS of the specimen was estimated [32, 38].
where, x is penetration gradient (ration between penetration resistant (N) and penetration depth (mm)); y is corresponding UCS.
2.8 CaCO3 content determination
Carbonate content in the treated specimen was determined by using the simplified device developed to measure the pressure of CO2 gas released when the cemented specimen is treated with HCl in closed system under constant volume and temperature [39]. Cemented column specimen was divided into sections along the height, and the samples from the sections at the depths of 1 cm, 3 cm and 5 cm (measured from the surface) were carefully separated, ensuring an accurate representation of cemented soil at that depth. Dry mass of the separated sample was measured, and the sample was placed into the calcimeter flask. 20 mL of HCl (3 mol/L) was placed in small plastic vials and set into the calcimeter flask without spattering the specimen. Subsequently, HCl was allowed to react with specimen in the closed system until the digital manometer (connected with the system) read a constant pressure. From the calibration curve developed between the pressure and CaCO3 content, the carbonate content was estimated, hence the percentage of mass of CaCO3 was determined.
3 Results and discussions
3.1 Biological response
3.1.1 Indigenous isolates
Among the isolated colonies, only three strains showed the pH increase and colour change in cresol red indicator, suggesting the urease potential. The strains were characterized as Lysinibacillus xylanilyticus, Viridibacillus arvi and Sporosarcina sp. by the 16S rRNA gene sequence analysis (the authenticate phylograms of the bacteria are presented as supplementary file). From the preliminary experiment results (Fig. 4), Lysinibacillus xylanilyticus culture was chosen based on the highest urease production and investigated in detail for MICP application. Lysinibacillus xylanilyticus is a gram positive, rod shaped bacteria (average length of 8–10 μm; diameter of 0.5–0.6 μm). It is worth noting that this is the first paper reporting the urease and MICP potential of the Lysinibacillus xylanilyticus.
3.1.2 Effect of temperature on growth and urease performance
Biomass concentration was used as the bacteria growth indicator based on the turbidity of the bacteria culture. The optical density (OD600) of Lysinibacillus xylanilyticus culture was measured by using the spectrophotometer at a wave length of 600 nm. The influence of temperature in bacteria growth was investigated by placing the bacteria culture in shaking incubator at temperatures ranging from 10 to 50 °C. The growth curves are presented in Fig. 5. The initial pH of the culture medium was relatively neutral (7.1–7.3). At the temperatures from 10 to 30 °C, biomass concentration increased initially up to 48–96 h and remained relatively stable afterwards. The optimal bacteria growth decreases with increase in temperature, and the growth is negligible at the temperatures above 40 °C.
Temperature and pH are the most governing factors of activity of bacterial urease [23]. Urease activities under different temperatures (10–50 °C) are provided in Fig. 6. One unit of activity can be defined as the amount of urea (μmol) degraded per minute by 1 mL bacterial culture enriched under the study conditions. It should be noted that relatively a bell-shape relationship between urease activity and temperature was obtained. Urease activity of Lysinibacillus xylanilyticus is relatively higher within the temperature range 20–25 °C (ranged between 1.5 and 3 μmol/min/mL). The urease activity reached the optimum value at 25 °C (3 μmol/min/mL), above which the activity dropped significantly and became negligible. The urease activities of the bacteria at 15 °C and 10 °C are 0.75 μmol/min/mL and 0.2 μmol/min/mL respectively, and below 5 °C, the activity becomes insignificant.
The long-term stability of bacterial urease is very essential for the adequate carbonation during treatment period under the minimal supply of bacteria cells. Urease is a molecule composed of protein subunits, that could be typically broken down into amino acids by cellular enzyme called protease (proteolysis) [40, 41]. The stability of bacterial urease with the time is shown in Fig. 7. The results indicate that the urease activity of Lysinibacillus xylanilyticus remains relatively stable within 15–25 °C against proteolysis. However, the urease activity became negligible within the short period at higher temperature (30 °C), suggesting that the bonds between protein subunits weaken at higher temperature and tended to break down quickly.
3.1.3 Effect of pH in urease performance
Effect of pH conditions in urease activity of Lysinibacillus xylanilyticus is illustrated in Fig. 8. The results show that the highest urease activity was found to be within the pH range from 7 to 8 (weak alkali conditions). Relatively lower urease activity was found under acidic (less than pH 6) and alkali (at pH 9) conditions, as bacterial urease underwent significant denaturing in severe acidic and alkali conditions.
3.1.4 Enzyme location
The whole cell culture medium was centrifuged (8000 rpm, 10 °C, 5 min) to separate the cell pellets from the culture supernatant. The separated cell pellets were washed and resuspended into same volume of autoclaved distilled water, followed by urease activity test was performed separately on cell medium (cells + distilled water) and supernatant. The urease activity of cell medium was very close to that of whole cell culture medium, and a negligible activity was observed in supernatant. The results suggest that the urease enzyme of Lysinibacillus xylanilyticus is not segregated into extracellular medium but localized completely in or on the cell membrane, indicating urease of Lysinibacillus xylanilyticus is cytoplasmic enzyme. This is similar to that reported to most of the urease of the species including Sporosarcina and Pararhodobacter sp. [27, 41].
3.1.5 Discussion
Numerous investigators have studied the effect of temperature on the rate of urea hydrolysis of many soil bacteria [23, 27, 30, 40]. In general, their results have indicated that the rate of urea hydrolysis increases with increasing temperature, suggesting that the urease of most of the soil bacteria is not heat inactivated (up to the temperature ranging 45–60 °C). For an example, urease activity of Sporosarcina Pasteurii increased proportionally with temperature between 25 °C and 60 °C [24]. The similar tendency is reported for Pararhodobacter sp. [27], Deleya venusta [42] and Strep-tococcus salivarius [43]. It is worth to note that the Lysinibacillus xylanilyticus reported herein exhibited a distinct behavior regarding urease activity, in which the urease enzyme is highly heat inactivated; urease is not stable even at 30 °C. On the other hand, the urease enzyme is more stable and consistently performs for long period at lower temperatures below 30 °C (Fig. 7). Theoretically, the activation energy for a specific enzyme-catalyzed reaction is constant [30]. Since the mean activation energy of most of the bacterial urease is high, bacteria could effectively catalyst the hydrolysis of urea at high temperatures (above 30 °C), resulting negligible urease potential at lower temperatures. Since the Lysinibacillus xylanilyticus was isolated from subarctic cold region, it exhibited high urease potential at temperatures below 30 °C, suggesting low energy requirement for enzyme-catalyzed reaction. However, urease activity of the bacteria becomes negligible below 5 °C, suggesting that the supply energy below 5 °C is deficient to activate the enzyme catalyzation. Therefore, application of the species reported herein is limited at the temperatures close to or below the freezing point of water.
Different soil bacteria has different tolerance towards pH conditions. Some ureolytic bacteria including Bacillus cereus show higher activity at around pH 4 compared to the neutral pH conditions suggesting acidic urease compatibility [44]. Also, the bacteria (for example Helicobacter pylori) living in harsh acidic environment produce more urease enzyme at acidic conditions [45]. In fact, urease activity of those bacteria is related to their stress response to survive at acidic condition, wherein hydrolysis of urea would neutralize the acidic environment leading to the bacterial survival. On the other hand, urease enzyme of some bacteria tends to be more active in neutral to alkaline environmental conditions. Lysinibacillus xylanilyticus reported in this paper showed the highest activity at weak alkali conditions. Similar phenomena could be seen on certain microbial urease like Sporosarcina pasteurii [23, 40], Pararhodobacter sp. [27] and Klebsiella aerogenes [44], those have the optimum activity under weak alkaline conditions.
3.2 Soil column test
3.2.1 Behavior of uniformly graded sands
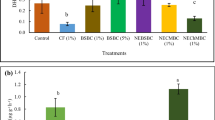
The effect of MICP in slope soil was examined by comparing the behavior of three uniformly graded sands with different particle sizes. All the columns specimens were treated exactly under same physical (25 °C) and chemical conditions (14 cementation injections; 2 bacterial injections) by surface percolation as explained in previous section. The entire column of all type of soils were successfully cemented, and the cemented profiles are presented in Fig. 9. Figure 10 presents the UCS of the different soils with respect to the depth. The results indicate that the UCS values increase with the decrease in particle size in the uniformly graded sands. The average UCS estimated close to the surface of Mizunami, Mikawa and Toyoura sands (D50 of 1.6 mm, 0.87 mm and 0.2 mm) are 1.82 MPa, 2.67 MPa and 3.98 MPa respectively. The UCS values and the calcium carbonate content (Fig. 11) of coarse and find sands reported herein are in a good agreement with the results reported by Cheng and Cord-Ruwisch [3]. Basically, coarse sands have high permeability that leads to the high liquid infiltration compared to the fine sands. The calcium ion concentrations of the drainage measured during the treatment (Fig. 12) suggest that more than 80% of the un-reacted cementation solutions have reached the bottom in the coarse sand columns (Mizunami and Mikawa) due to the high infiltration rate facilitated by the large pore spaces.
The UCS of all the uniformly graded sands increases with the depth of the sample. In coarse sand columns, the UCS and CaCO3 content are relatively constant from the surface to the middle and then slightly increase with the depth. A similar observation in coarse sand has been reported by Cheng and Cord-Ruwisch [3]. In contrast, fine sand exhibits significant improvement in UCS with the depth, which could be due to the increased capillary effect along the sample depth. The higher capillary forces in fine sand tended to retain certain amount of cementation solutions close to particle contacts, resulting higher deposition of calcium carbonate and exhibiting higher UCS at the bottom sample compared to that at top.
3.2.2 Behavior of slope soil
The slope soil showed a distinct behavior compared to the behaviors of uniformly graded sands. The average particle size of Toyoura sand and slope soil are relatively similar (0.20 mm and 0.23 mm respectively). Also, both soils exhibited that around 80–90% of the calcium ions in cementation solution have been already utilized by the time it reached the bottom (Fig. 12). However, treated soils exhibited a significant difference regarding their UCS (Fig. 10) and CaCO3 content (Fig. 11). The UCS of the slope soil estimated close to the surface is 14.98 kPa, which is around 3.75 times higher than the UCS of cemented Toyoura sand. There was no difference in the treatment process for the above specimens; therefore, the reason for the remarkable UCS achieved in slope soil could be due to its grain size distribution. Slope soil is a well graded sand consisting very fine sand content of 12%, leading to effective packing and cementation in the matrix. It was also observed that the infiltration rate of cementation solution decreased with the number of injections, which would be the result of the reduction in permeability due to the cementation at pore spaces.
In contrast to the UCS and CaCO3 profile of uniformly graded sand columns, the UCS and carbonate content of slope soil decrease with the depth (Figs. 10 and 11). The higher cementation at the top of the column specimen is presumed to be due to the biological filtration. The bacteria were applied into specimen from top and allowed to flow through the soil. As the bacteria travel through smaller pore space of the slope soil, they might be filtered through the soil with a reduction of bacteria concentration along the path. Therefore, a larger concentration of bacteria cells would retain at the top of the column compared to the bottom. The distribution of carbonate cementation observed in slope soil was similar to those reported by Cheng and Cord-Ruwisch [3], Feng and Montoya [11] and Martinez et al. [46]. Furthermore, a very less bacterial turbidity was detected at the outlet of slope soil column, suggesting that most of the cells are immobilized in the soil matrix by the filtration. It has been reported that the cementation profiles are generally determined by the bacteria distribution in the specimen [46]. The uniformity of the precipitated calcium carbonate along the soil specimens is further discussed in the subsequent section.
3.2.3 Microscale properties of MICP treated soils
The precipitation characteristics of MICP treated soils were investigated in microscale using SEM (Scanning electron microscopy). All the representative samples used for the SEM observation were obtained from 1 cm depth of the treated columns. The SEM images of uniformly graded sands are shown in Fig. 13. The images of MICP treated sands show calcium carbonate precipitated at particle contacts (contact cementing) and slightly coating particle surfaces. As the particle size of sand reduces from 1.6 mm to 0.2 mm, the images of the MICP treated sands show an increase in precipitated carbonate content (consistent with the carbonate content measurement of sands presented in Fig. 11).
The number of particles contact highly influences the effectiveness of MICP. Generally, bacteria cells prefer to position themselves in smaller surface features (such as near particle–particle contacts) compared to the particle surface, which is due to the reduced shear and a higher availability of nutrients at the particle contacts [4]. Therefore, the calcium carbonate cement preferentially precipitates at the particle contacts while coating the soil grains during the treatment. Due to the higher number of particle contacts in fine Toyoura sand, the rate of calcium carbonate precipitation was higher compared to that in coarse sands (Fig. 13). It should be noted that the matrix supporting is enabled by filling the void space with or without contacting the soil particles (i.e. grow from particle surface into pore space to create bridges between particles), when the soils are treated in saturated conditions [4, 5, 11]. Well coated grains with matrix supporting by calcite crystals are also often observed by many researchers in medium to coarse sands treated under saturated conditions [2, 5, 11, 47]. It is worth noting that the microstructure reported herein for the specimens treated under percolation condition slightly differ from that reported for saturated conditions. Since the surface percolations method is achieved with the aid of gravitational and capillary forces, contribution of matrix supporting is limited. The growth of the crystals from the particle surface into void space is not observed in uniformly graded sands treated by surface percolation (Fig. 13).
Figure 14 presents the SEM image of MICP treated slope soil (Fig. 14a shows the untreated soil). Based on the shape of the CaCO3 crystal, the precipitated morphology in slope soil would be calcite (typical rhombohedral crystals). It can be clearly seen that the sand particles ranging from coarse to fine form an effective matrix in slope soil for the MICP (Fig. 14b). It is also worth noting that presence of fine particles could change the microstructure of sand skeleton and govern the behavior significantly. Most of the sand particles are in contact with some of the fine particles between them, and the rest filled the void spaces (Fig. 14b). Also, some of the fine particles are cemented both to each other and to the sand particles (Fig. 14c). The presence of fine content (12%) in slope soil significantly increases the number of particle contacts by bonding with the sand grains. It can be observed that the fine content plays a very important role in matrix supporting by enabling intermediate supports to form bridges among the carbonate crystals that have grown in void spaces (Fig. 14c), thus, strengthening the force chain of the treated matrix. It should also be stated here that the MICP in very fine soils are limited due to the limited rate of permeability that generally takes very longer time to infiltrate the reactants [13], and free passage of the bacteria might be inhibited due to the small pore throat size of fine soils [47].
3.3 Slope model solidification test
Slope model test is the scale-up version of soil column tests. Because the small columns are limited by confinement and boundary effects, the model test was additionally performed to demonstrate the feasibility of slope soil stabilization. After the 14 days of treatment, the slope was flushed with about 5 L of tap water to remove all soluble salts, followed by, slope specimen was carefully removed from the mold and submerged into the water bath to eliminate the uncemented aggregates. The cemented profile is presented in Fig. 15. The observation indicates that more than 80% of the soil was successfully cemented, and a stiff layer of around 3–4 cm along the entire surface was achieved. However, cementation was not observed within the zone below the top slope (Fig. 15), remained as untreated soil. The surface UCS was used as the indicator to evaluate the improvement of slope, besides the UCS estimated by the needle penetration tests are illustrated in Fig. 16. The results show that the bio-cemented slope surface had considerable UCS between 2 and 8 MPa, and relatively lower UCS values were obtained below the surface of the slope.
3.3.1 Uniformity of cementation
It is highly necessary to study uniformity of precipitated calcium carbonate along laterally (t-z axis) and vertically (x–y axis) in slope, as the MICP in soils is achieved by biochemical injections. Before the measurements, it was anticipated that relatively a homogeneous cementation could be attained along the surface (t-z) by the injection method. However, Fig. 16 suggests that the surface UCS increases along the distance (t) measured from the top slope, and the highest surface UCS was obtained near bottom slope. The average UCS at the bottom slope was about 7 MPa, whereas that of top is only 2 MPa. This variation along the surface could be attributed to the localization of bacteria cells. The consecutive injections of cementation solution (every 24 h) i.e. propagated flow-lines would possibly transport the bacteria cells towards downslope, results increase in cell concentration in the direction of downslope; thus, more or less of the bacteria cells will in turn increase or decrease the cementation and UCS. The Precipitated carbonate profile is not uniform along the slope depth (x–y) similar to that observed in column specimens, suggesting higher calcium carbonate content near to the injection port. The spatial distribution of calcium carbonate generally follows the trend of microbe distributions along the specimen length/depth [46]. As previously discussed, higher concentration of bacteria cells was filtered at the top zone, leading to higher deposition of carbonate content near the injection surface. In order to confirm this postulation, soil samples were collected from three depths of slope after performing the biological injection, and the number of existing bacteria cells were measured by similar method used for isolation of bacteria. The measured locations are indicated clearly in Fig. 3. The results (Fig. 17) suggest that the distribution of bacteria in slope soil drastically decreases with the depth of slope i.e. the number of cells per unit gram soil at 1 cm below the slope surface and at the bottom slope are 1.4 × 106 and 5 × 103 respectively. Thus, it has been confirmed that most of the bacteria cells were filtered in surface zone, resulting high cementation at the surface zone.
Distribution of bacteria cells along the depth of the slope (locations A, B and C are indicated in Fig. 3)
In addition, surface zone closest to the injection port is generally in exposure to higher concentrated reactants than the bottom zone [1]; therefore, higher carbonate content was precipitated at surface zone. It also has been reported that the amount of urease, precipitation rate and nutrient concentrations also have a great influence in carbonate distribution and must be maintained at their minimum to achieve a homogeneous cementation along the soil columns [13, 47].
The measurements indicate that the slope soil consists of very small amount (0.22–0.25%) natural carbonate content. As reported by Mortensen et al. [13], the soil particles consisted of carbonate content could provide ideal surfaces and nucleation sites for the additional precipitation of carbonate crystals. At the same time, the residual soil would consist of soil particles in broad range of shapes, irregularities and with varying surface roughness, which could provide additional effects in precipitation of carbonate. Future works should clearly address the effect of particle shapes and surface roughness to improve the understanding on the behavior of residual soils.
The threshold calcium carbonate content required to provide measurable UCS of slope soil was found to be around 3%. The carbonate content reported herein is in a good agreement with the minimum carbonate content (3.5%, for the sand with D50 of 165μm) reported by Whiffin et al. [1]. Soil cemented below the threshold carbonate content exhibited an unsolidified loose matrix which was similar to the untreated soil. However, small carbonate crystals on the particle surface were evidenced through SEM analysis. At the same time, Feng and Montoya [11] and Lin et al. [5] have reported that the minimum carbonate content required to increase the strength of sands (D50 of 0.22 mm and 0.33 mm respectively) was around 1%. Actually, the threshold carbonate content of a soil is related to the number of particle contacts and grain size. Due to the increased number of particle contacts in slope soil, relatively a higher amount of threshold carbonate content (3%) (compared to that reported by Feng and Montoya [11] and Lin et al. [5]) was required to provide the measurable strength.
Figure 18 presents a compilation of all UCS results obtained versus the measured calcium carbonate content. The UCS increases during MICP improvement as calcium carbonate precipitates and cements the soil particles, as a result of void space reduction. It is also clear that the UCS increases exponentially with the increase in calcium carbonate content, similar to that reported in previous studies [2, 6, 32].
3.3.2 Field implication and associated limitations
To date, most of the studies of MICP have been extensively investigated under saturated or water-logged environmental conditions [1, 5, 6, 10, 47], in which bacterial and cementation medias were injected to the specimen by a saturated flow with a constant flow rate using peristaltic pump. Pumping method has often resulted a heterogeneously cemented profile in soil columns, suggesting a higher precipitation level at the influent column. Recently, Li et al. [48] have proposed a rotating soak method to promote the uniformity of cementation in specimen by facilitating more nutrition supply and air replenishment within the soil for effective bacterial performance. However, in the real field, it would be a challenge to maintain the saturated or submerged flow conditions during the treatment period, which requires hydraulic injection of cementation/biological solutions, extraction of effluent solution and heavy machinery systems. Therefore, it is an essential to introduce alternative MICP approaches to produce carbonate cement with high efficacy with desirable cost minimization. The simple surface percolation technique demonstrated in this study is more suitable for field application, which eliminates the destruction of existing soil structure and reduces the costs of required labour and machinery. However, it would be more effective, if the surface percolation is promoted to spraying instead of injections, which is left for the future work to be performed in the subsequent phase of this study.
In civil engineering applications such as slope surface stabilizations, it is highly important to immobilize the ureolytic bacteria within the target surface zones, so that sufficient carbonate can be precipitated throughout the required zone. The experimental observations discussed in this paper suggest well immobilization of bacteria only within the surface zone, and the particle size distribution of slope soil limits the distribution of bacteria to more deeper depths. Therefore, it is appropriate to recommend the technique for slope surface stabilization i.e. stabilizing the slope by enhancing the surface cover condition of the slope and promoting high aggregate stability at the surface zone, which could be enabled against surface erosion. The results also indicate, treating the soil by surface percolation method could strengthen the soil significantly by forming more effective crystals at free-draining environmental conditions and can be highly applicable on unsaturated or partially saturated natural slopes and embankments.
In the injection method investigated herein, grain size distribution highly governs the efficiency of MICP. Fine sands, well graded soils and soils consisting of certain amount of fine particles have less permeability (compared to the coarse soils) leading to low infiltration rate, that often increases the risk regarding local clogging. In order to avoid the risk of clogging, Whiffin et al. [1] have suggested a faster injection/flow rates of reactants by allowing less reaction time along the path. Afterwards, Cheng and Cord-Ruwisch [3] have reported the observation of non-clogged fine and coarse sand columns treated under higher infiltration rates (7–28.5 cm/min). They have also reported that severe clogging occurred in fine sand when it was treated under low infiltration rate less than 0.25 cm/min. Therefore, a moderate infiltration rate of 2–4 mL/min was maintained during the treatment period (no clogging occurred during the treatment).
4 Conclusions
The findings indicate that the bacteria (Lysinibacillus xylanilyticus) isolated from the subarctic cold region have a significant potential to produce urease enzyme at temperatures 15–25 °C, suggesting low-temperature urease compatibility. On the other hand, the growth and the urease production of the bacteria is limited at the temperatures above 30 °C. In the neutral-weak alkaline environmental conditions, urease is more stable and consistently performs. It is also found that the urease is not segregated into the extracellular medium indicating that bacterial urease is a cytoplasmic enzyme. On the whole, the novel isolate exhibits a significant impact for MICP applications in subarctic cold climatic regions.
The effect of carbonate precipitation on the process of strengthening in slope soil was investigated by comparing the behaviour of three uniformly graded sands. Microstructure of the soils treated by surface percolation shows the formation of effective crystals at particle contacts. Presence of fine content governs the behavior of residual slope soil significantly. It increases the number of particle contacts by bonding with the sand grains and participates in the force chain of the treated matrix. The fine content provides the matrix support effectively by facilitating intermediate supports to form bridges between carbonate crystals. However, grain size distribution tends to filter more bacteria at surface zone of slope, results high cementation at the surface level and relatively a long linear reduction in carbonate precipitation along the profile is observed. This preliminary investigation suggests that the technique can be implemented for stabilizing the slope by enhancing the cover condition of the slope, and the results obtained from this investigation would be highly beneficial to promote the study to the large scale.
References
Whiffin VS, van Paassen LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24:417–423. https://doi.org/10.1080/01490450701436505
Amarakoon GGNN, Kawasaki S (2018) Factors affecting sand solidification using MICP with Pararhodobacter sp. Mater Trans 59:72–81. https://doi.org/10.2320/matertrans.M-M2017849
Cheng L, Cord-Ruwisch R (2014) Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol J 31:396–406. https://doi.org/10.1080/01490451.2013.836579
DeJong JT, Mortensen BM, Martinez BC, Nelson DC (2010) Bio-mediated soil improvement. Ecol Eng 36:197–210. https://doi.org/10.1016/j.ecoleng.2008.12.029
Lin H, Suleiman MT, Brown DG, Kavazanjian E (2016) Mechanical behavior of sands treated by microbially induced carbonate precipitation. J Geotech Geoenviron Eng 142:04015066-1–13. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001383
van Paassen LA, Ghose R, van der Linden TJM et al (2010) Quantifying biomediated ground improvement by ureolysis: large-scale biogrout experiment. J Geotech Geoenviron Eng 136:1721–1728. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000382
Montoya BM, DeJong JT, Boulanger RW (2013) Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. Géotechnique 63:302–312. https://doi.org/10.1680/geot.sip13.p.019
Gomez MG, Anderson CM, Graddy CMR et al (2017) Large-scale comparison of bioaugmentation and biostimulation approaches for biocementation of sands. J Geotech Geoenviron Eng 143:04016124. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001640
Danjo T, Kawasaki S (2016) Microbially induced sand cementation method using Pararhodobacter sp. Strain SO1, inspired by Beachrock formation mechanism. Mater Trans 57:428–437. https://doi.org/10.2320/matertrans.M-M2015842
Feng K, Montoya BM (2017) Quantifying level of microbial-induced cementation for cyclically loaded sand. J Geotech Geoenviron Eng 143:06017005. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001682
Feng K, Montoya BM (2016) Influence of confinement and cementation level on the behavior of microbial-induced calcite precipitated sands under monotonic drained loading. J Geotech Geoenviron Eng 142:04015057. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001379
van Paassen LA (2011) Bio-Mediated Ground Improvement: From Laboratory Experiment to Pilot Applications. Geo-frontiers 2011. American Society of Civil Engineers, Reston, pp 4099–4108
Mortensen BM, Haber MJ, Dejong JT et al (2011) Effects of environmental factors on microbial induced calcium carbonate precipitation. J Appl Microbiol 111:338–349. https://doi.org/10.1111/j.1365-2672.2011.05065.x
Soon NW, Lee LM, Khun TC, Ling HS (2014) Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. J Geotech Geoenviron Eng 140:04014006. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001089
Osinubi KJ, Eberemu AO, Gadzama EW, Ijimdiya TS (2019) Plasticity characteristics of lateritic soil treated with Sporosarcina pasteurii in microbial-induced calcite precipitation application. SN Appl Sci 1:829. https://doi.org/10.1007/s42452-019-0868-7
Li C, Yao D, Liu S et al (2018) Improvement of geomechanical properties of bio-remediated Aeolian sand. Geomicrobiol J 35:132–140. https://doi.org/10.1080/01490451.2017.1338798
Fujita Y, Grant Ferris F, Daniel Lawson R et al (2000) Calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiol J 17:305–318. https://doi.org/10.1080/782198884
Gomez MG, Martinez BC, DeJong JT et al (2015) Field-scale bio-cementation tests to improve sands. Proc Inst Civ Eng - Gr Improv 168:206–216. https://doi.org/10.1680/grim.13.00052
Gat D, Ronen Z, Tsesarsky M (2016) Soil bacteria population dynamics following stimulation for ureolytic microbial-induced CaCO3 precipitation. Environ Sci Technol 50:616–624. https://doi.org/10.1021/acs.est.5b04033
Sensoy T, Bozbeyoglu NN, Dogan NM, et al (2017) Characterization of calcium carbonate produced by ureolytic bacteria (Sporocarcina pasteurii ATCC 6453 and Bacillus aerius U2) and effect of environmental conditions on production of calcium carbonate. In: 15th International conference on environmental science and technology. Rhodes, Greece
DeJong JT, Martinez BC, Mortensen BM, et al (2009) Upscaling of bio-mediated soil improvement upscaling of bio-mediated soil improvement. In: 17th International conference on soil mechanics and geotechnical engineering. Netherlands, pp 2300–2303
Yang Y, Zhu G, Wang G et al (2016) Robust glucose oxidase with a Fe3O4@C-silica nanohybrid structure. J Mater Chem B 4:4726–4731. https://doi.org/10.1039/c6tb01355d
Omoregie AI, Khoshdelnezamiha G, Senian N et al (2017) Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol Eng 109:65–75. https://doi.org/10.1016/j.ecoleng.2017.09.012
Whiffin VS (2004) Microbial CaCO3 precipitation for the production of biocement. PhD thesis, Murdoch University, Australia. https://researchrepository.murdoch.edu.au/id/eprint/399/
Omoregie AI, Palombo EA, Ong DEL, Nissom PM (2019) Biocementation of sand by Sporosarcina pasteurii strain and technical-grade cementation reagents through surface percolation treatment method. Constr Build Mater 228:116828. https://doi.org/10.1016/j.conbuildmat.2019.116828
Cheng L, Cord-Ruwisch R, Shahin MA (2013) Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can Geotech J 50:81–90. https://doi.org/10.1139/cgj-2012-0023
Fujita M, Nakashima K, Achal V, Kawasaki S (2017) Whole-cell evaluation of urease activity of Pararhodobacter sp. isolated from peripheral beachrock. Biochem Eng J 124:1–5. https://doi.org/10.1016/j.bej.2017.04.004
Stabnikov V, Naeimi M, Ivanov V, Chu J (2011) Formation of water-impermeable crust on sand surface using biocement. Cem Concr Res 41:1143–1149. https://doi.org/10.1016/j.cemconres.2011.06.017
Chu J, Stabnikov V, Ivanov V (2012) Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol J 29:544–549. https://doi.org/10.1080/01490451.2011.592929
Moyo CC, Kissel DE, Cabrera ML (1989) Temperature effects on soil urease activity. Soil Biol Biochem 21:935–938. https://doi.org/10.1016/0038-0717(89)90083-7
ASTM D2487–17 (2017) Standard practice for classification of soils for engineering purposes (unified soil classification system). ASTM International, West Conshohocken
Gowthaman S, Mitsuyama S, Nakashima K et al (2019) Biogeotechnical approach for slope soil stabilization using locally isolated bacteria and inexpensive low-grade chemicals: a feasibility study on Hokkaido expressway soil, Japan. Soils Found 59:484–499. https://doi.org/10.1016/j.sandf.2018.12.010
Bolleter WT, Bushman CJ, Tidwell PW (1961) Spectrophotometric determination of ammonia as indophenol. Anal Chem 33:592–594. https://doi.org/10.1021/ac60172a034
Japan Road Association (2009) Road earthworks: cut earthworks, earthquake, slope stabilization guidelines. Tokyo, Japan. ISBN-13: 978-4889504156 (in Japanese)
Gowthaman S, Mitsuyama S, Nakashima K et al (2019) Microbial induced slope surface stabilization using industrial-grade chemicals: a preliminary laboratory study. Int J GEOMATE 17:110–116. https://doi.org/10.21660/2019.60.8150
JGS (Japanese Geotechnical Society) (2012) Method for needle penetration test (JGS: 3431–2012). In: Japanese standards and explanations of geotechnical and geoenvironmental investigation methods, No. 1. JGS Publication. Tokyo, pp 426–432 (in Japanese)
Ulusay R (2014) The ISRM suggested methods for rock characterization, testing and monitoring: 2007–2014. Springer, Berlin
Fukue M, Ono S-I, Sato Y (2011) Cementation of sands due to microbiologically-induced carbonate precipitation. Soils Found 51:83–93. https://doi.org/10.3208/sandf.51.83
Fukue M, Nakamura T, Kato Y (2001) A method for determining carbonate content for soils and evaluation of the results. Soils Found 49–2:9–12 (in Japanese)
Ciurli S, Marzadori C, Benini S et al (1996) Urease from the soil bacterium Bacillus pasteurii: immobilization on Ca- polygalacturonate. Soil Biol Biochem 28:811–817. https://doi.org/10.1016/0038-0717(96)00020-X
Mobley HL, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases. Microbiol Rev 59:451–480
Jahns T (1992) Urea uptake by the marine bacterium Deleya venusta HG1. J Gen Microbiol 138:1815–1820. https://doi.org/10.1099/00221287-138-9-1815
Chen YYM, Anne Clancy K, Burne RA (1996) Streptococcus salivarius urease: genetic and biochemical characterization and expression in a dental plaque streptococcus. Infect Immun 64:585–592
Phang IRK, Chan YS, Wong KS, Lau SY (2018) Isolation and characterization of urease-producing bacteria from tropical peat. Biocatal Agric Biotechnol 13:168–175. https://doi.org/10.1016/j.bcab.2017.12.006
Bauerfeind P, Garner R, Dunn BE, Mobley HL (1997) Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut 40:25–30
Martinez BC, DeJong JT, Ginn TR et al (2013) Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J Geotech Geoenviron Eng 139:587–598. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000787
Cheng L, Shahin MA, Mujah D (2016) Influence of key environmental conditions on microbially induced cementation for soil stabilization. J Geotech Geoenviron Eng 143:04016083. https://doi.org/10.1061/(asce)gt.1943-5606.0001586
Li H, Li C, Zhou T et al (2018) An improved rotating soak method for MICP-treated fine sand in specimen preparation. Geotech Test J 41:20170109. https://doi.org/10.1520/GTJ20170109
Acknowledgements
The authors highly appreciate the Hokkaido Office, East Nippon Expressway Company Limited, Sapporo, Hokkaido, Japan for providing the research opportunity and the continuous supports in sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gowthaman, S., Iki, T., Nakashima, K. et al. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl. Sci. 1, 1480 (2019). https://doi.org/10.1007/s42452-019-1508-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1508-y