Abstract
Background
Chronic inflammation plays an important role in the progression of vascular calcification (VC). This study was designed to explore the effects and underlying mechanisms of inflammation on VC in the radial arteries of patients with end-stage renal disease (ESRD) with arteriovenostomy.
Methods
Forty-eight ESRD patients were divided into control (n = 25) and inflammation groups (n = 23) according to plasma C-reactive protein (CRP) level. Surgically removed tissues from the radial arteries of patients receiving arteriovenostomy were used in this study. Alizarin Red S staining was used to examine calcium deposition. The expression of inflammation markers, bone structure-associated proteins and mammalian target of rapamycin complex1 (mTORC1) pathway-related proteins was assessed by immunohistochemical staining.
Results
The expression of tumor necrosis factor-α (TNF-α) and monocyte chemotactic protein-1 (MCP-1) was increased in the radial arteries of the inflammation group. Additionally, Alizarin Red S staining revealed a marked increase in calcium deposition in the inflammation group compared to controls. Further analysis by immunohistochemical staining demonstrated that the deposition was correlated with the increased expression of bone-associated proteins such as bone morphogenetic proteins-2 (BMP-2) and osteocalcin and collagen I, which suggested that inflammation induces osteogenic differentiation in vascular tissues and that osteogenic cells are the main cellular components involved in VC. Interestingly, there was a parallel increase in the expression of phosphorylated mTOR (p-mTOR) and pribosomal protein S6 kinase 1 (p-S6K1) in the inflammation group. Furthermore, mTORC1 pathway-related proteins were significantly associated with the enhanced expression of bone formation biomarkers.
Conclusions
Inflammation contributed to VC in the radial arteries of ESRD patients via the induction of osteogenic differentiation in vessel walls, which could be regulated by the activation of the mTORC1 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular calcification (VC) is a common complication of end-stage renal disease (ESRD) and is predictive of subsequent cardiovascular disease (CVD) and mortality [1]. Both traditional (old age, smoking, hypertension, hyperlipidemia and diabetes) and nontraditional (anemia, abnormal calcium/phosphorus metabolism, hyperhomocysteinemia, oxidative stress, and systemic inflammation) risk factors contribute to excessive and accelerated VC in ESRD patients on chronic dialysis, especially in patients receiving hemodialysis (HD) [2]. It is well recognized that chronic kidney disease (CKD) is a microinflammatory state [3]. Recent evidence demonstrates that many inflammatory markers and mediators, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP), are substantially increased in ESRD populations [4]. Furthermore, chronic systemic inflammation, a non-traditional risk factor, accelerates the progression of VC, which has been identified as an independent risk factor for the morbidity and mortality of cardiovascular disease (CVD) in ESRD patients [5, 6]. However, the pathogenesis of VC and chronic inflammation in ESRD remains largely unknown, and effective investigations are urgently needed.
Vascular calcification (VC) is a complicated pathological process that develops primarily within the tunica intima and tunica media [7]. In CKD patients, VC is known as artery medial calcification (AMC) and is characterized by amorphous minerals forming along one or more elastic lamellae of the medial layer [8]. Recent studies have established that VC in CKD is a highly regulated cell-mediated process that involves the entry of vascular cells, mainly the medial vascular smooth muscle cells (VSMCs), into a transdifferentiation program of osteogenesis during which numerous key regulators of mineralized matrix and osteogenic proteins are produced, thereby promoting the process of VC [9, 10]. Accumulating evidence has demonstrated that chronic inflammation is involved in the pathogenesis of osteogenic transition of vascular cells in ESRD [11, 12]. In vitro results showed that inflammatory cytokines can promote the calcification of VSMCs, in part through the activation of Wnt/β-catenin signaling, since the Wnt/β-catenin pathway is necessary for the commencement of osteoblast differentiation [13].
Moreover, inflammation promotes the process of endothelial to mesenchymal transition, leading to osteogenic gene expression and cytokine production by endothelial cells [14]. Therefore, in response to inflammatory stimuli, vascular cells express and/or release many regulators of bone formation and bone-associated proteins, such as bone morphogenetic protein-2 (BMP-2), osteocalcin (OCN), alkaline phosphatase (ALP), and collagen I. In contrast, the expression of vascular markers such as α-smooth muscle cells (α-SMA) and collagen IV is reduced, ultimately transforming vessel cells into osteoblast-like cells [15, 16]. However, the precise mechanisms that cause the osteogenic phenotype in calcified vessels under inflammation are not completely understood.
Mammalian target of rapamycin (mTOR) is an atypical serine/threonine protein kinase with a predicted molecular weight of 289 kDa [17]. Zhao et al. report that in a rat model of chronic renal failure, mTOR signaling is activated in the aortic wall, partly through exposure to high phosphate levels, and that this activation greatly influences the pathogenesis of AMC involving the suppression of Klotho expression in the vasculature [18]. Reported Klotho functions include inhibition of local phosphate uptake, suppression of osteoblast-like differentiation of VSMCs, attenuation of matrix mineralization and calcification [19]. In addition, rapamycin, an inhibitor of mTOR complex1 (mTORC1), has been shown to regulate phosphate homeostasis and osteoblastic differentiation, which suggests that mTORC1 is an intriguing candidate as a regulator of osteogenesis [20]. Our previous studies showed that the mTORC1 pathway can be activated by inflammatory factors such as lipopolysaccharide (LPS) and IL-1β, and subsequently, it phosphorylates downstream proteins of p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1), which ultimately results in atherosclerosis and non-alcoholic fatty liver disease [21, 22]. However, the potential role of mTORC1 in the progression of VC, particularly in the modulation of osteogenesis in calcified vessels under chronic inflammation, has been not defined yet.
The present study was undertaken to investigate whether chronic systemic inflammation exacerbates VC in the radial arteries of ESRD patients with arteriovenostomy, and to elucidate the underlying mechanisms.
Materials and methods
Patient selection and clinical data
We recruited 48 ESRD patients from Nanjing Drum Tower Hospital, China, between July 2015 and December 2016. Patients with ESRD who were to undergo arteriovenostomy before hemodialysis were included in the study. Patients with acute infection, cancer, and/or chronic active hepatitis were excluded. The included patients were divided into two groups based on their plasma CRP level: control (CRP < 8.0 mg/l, n = 25) and inflammation (CRP ≥ 8.0 mg/l, n = 23).
Clinical biochemical tests
Blood samples were assayed to determine red blood cell (RBC) count and levels of CRP, hemoglobin (Hb), total protein (TP), albumin (ALB), glucose (GLU), blood urea nitrogen (BUN), serum creatinine (Scr), triglycerides (TGs), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), apolipoprotein A1 (ApoA1), ApoB, calcium (Ca), phosphate (P), and intact parathyroid hormone (iPTH) using an automatic biochemistry analyzer at the clinical chemistry center of the hospital.
Tissue processing
Tissues from the radial artery were obtained during radial-cephalic anastomosis surgery. The tissue sections were rinsed with saline and placed in 10% buffered formalin for 24 h. After treatment, representative sections of the grafts were obtained and embedded in paraffin.
Alizarin Red S staining
Paraffin-embedded tissues (4 µm) were dewaxed and hydrated in 70% alcohol. After rapid rinsing in distilled water, the tissue slices were stained with Alizarin Red S solution for 1 min. Positive results were observed with an orange–red color under a light microscope (200×).
Immunohistochemical staining
Paraffin-embedded sections (4 µm) underwent immunohistochemical staining. After deparaffinization, sections were placed in citrate-buffered solution (pH 6.0) and then heated for antigen retrieval. Endogenous peroxidase was blocked with 3% hydrogen peroxide, and nonspecific antibody binding was blocked with 10% goat serum. Subsequently, the sections were incubated with goat, rabbit or mouse anti-human primary antibodies against TNF-α (Santa Cruz Biotechnology, Santa Cruz, CA, USA), MCP-1 (Santa Cruz Biotechnology), BMP-2 (Santa Cruz Biotechnology), OCN (Santa Cruz Biotechnology), collagen I (Abcam, Cambridge, UK), phosphorylated mTOR (p-mTOR) (Abcam) and p-S6K1 (Abcam) overnight at 4 °C, followed by incubation with biotinylated secondary antibodies. Finally, a diaminobenzidine tetrahydrochloride substrate was used to develop the reaction. The results were observed under a light microscope (200×).
Immunohistochemistry image analysis
Image Pro Plus Software 6.0 (Media Cybernetics, Rockville, MD, USA) was used to semi-quantitatively measure the concentration of the immune staining. This procedure included six steps: discovering and surveying the section of interest; adjusting the optical densities; obtaining and preserving images; amending the background and background staining; configuration of the section of interest to determine the optical density; and, lastly, examining mean optical density.
Ethics statement
All studies were approved by the Ethical Committee of Nanjing Drum Tower Hospital. Each patient provided written informed consent for the use of their tissues for research purposes.
Statistical analysis
All data were analyzed with SPSS 16.0. Continuous variables were compared between the two groups with independent sample t test and Mann–Whitney U test. The correlation between the groups was determined with Spearman’s correlation and Pearson’s correlation. A difference was considered significant for p < 0.05.
Results
Basic clinical data of the two groups
As shown in Table 1, there were no differences in age, body weight, RBC, Hb, TP, ALB, GLU, BUN, Scr, TG, TC, LDL, HDL, ApoA1, ApoB, Ca, P, Ca × P, or iPTH (p > 0.05) between the control group and the inflammation group.
Local upregulation of inflammation in the artery was consistent with plasma CRP levels
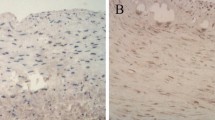
The expression of TNF-α and MCP-1, on immunohistochemical staining, was increased in the radial artery in the inflammation group, which indicated that local inflammation in the artery was upregulated, consistent with the observation of systemic inflammation (TNF-α 0.19 ± 0.02 vs. 0.37 ± 0.03, MCP-1 0.24 ± 0.05 vs. 0.55 ± 0.07) (Fig. 1).
Inflammation increased inflammatory cytokine expression in the radial arteries. a The local inflammation status in the radial artery was examined by immunohistochemical staining. The positive areas are stained brown in cross-sections of radial arteries (original magnification × 200). b Values of the semiquantitative analysis for the positive areas are expressed as mean ± SD in each group. *p < 0.05 vs. control
Inflammation induced calcified plaque deposition in radial arteries
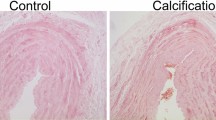
To evaluate the effect of inflammation on the progression of VC, we assessed calcified plaque formation by Alizarin Red S staining. There was a significant increase in calcified depositions in the radial arteries of the inflammation group compared to the control group (0.48 ± 0.19 vs. 10.12 ± 3.88) (Fig. 2).
Inflammation promoted calcified plaque deposition in the radial arteries. a The calcification was examined by Alizarin red S staining, and calcium deposits are stained orange-red (original magnification × 200). b Values of the semiquantitative analysis for the positive areas are expressed as mean ± SD in each group. *p < 0.001 vs. control
Inflammation accelerated VC by contributing to the transformation of vascular tissue into the osteogenic phenotype
To explore the potential mechanisms of VC in the context of inflammation, we evaluated the effects of inflammation on the expression of bone formation biomarkers such as BMP-2, OCN and collagen I in the radial arteries during VC progression. As shown by immunohistochemical staining, the expression levels of BMP-2, OCN and collagen I protein were significantly increased in the inflammation group compared to the control group (BMP-2 0.27 ± 0.07 vs. 0.41 ± 0.03, OCN 0.14 ± 0.03 vs. 0.25 ± 0.05, collagen I 0.54 ± 0.15 vs. 1.64 ± 0.28) (Fig. 3). Accumulating evidence has demonstrated that certain stimulations can induce the dedifferentiation of VSMCs from the contractile phenotype to an osteogenic phenotype, which results in the production of mineralized matrix and osteogenic proteins and promotes the process of VC. Our data suggested that inflammation induces osteogenic differentiation in vascular tissues, thereby accelerating the progression of VC.
Inflammation increased the expression of the bone formation-associated proteins in the radial arteries. a The bone morphogenetic biomarkers such as BMP-2, OCN and collagen I were checked by immunohistochemical staining. The positive areas are stained as brown in cross-sections of radial arteries (original magnification × 200). b Values of the semiquantitative analysis for the positive areas are expressed as mean ± SD in each group. *p < 0.05 vs. control, **p < 0.01 vs. control
Activation of the mTORC1 pathway was closely associated with inflammation-induced osteogenic transition in the radial arteries
To determine whether mTORC1 signaling modulates vascular phenotypic transition, immunohistochemical staining was used to examine the expression of mTORC1 pathway-related proteins. As shown in Fig. 4, the levels of p-mTOR and p-S6K1 were decreased compared to the inflammation group (p-mTOR 0.23 ± 0.07 vs. 1.29 ± 0.22, p-S6K1 0.16 ± 0.03 vs. 0.24 ± 0.03). In addition, correlation analysis demonstrated that p-mTOR and p-S6K1 were positively associated with the expression of BMP-2, OCN and collagen I (Fig. 5). These findings suggest that activation of the mTORC1 pathway under inflammatory stress may be closely associated with the formation of bone morphogenetic proteins in the vessels.
Inflammation induced the activation of the mTORC1 pathway. a The protein expression of p-mTOR and p-S6K1 was measured by immunohistochemical staining. The positive areas are stained brown in the radial artery cross-sections (original magnification × 200). b Values for the semiquantitative analysis of the positive areas are expressed as mean ± SD in each group. *p < 0.05 vs. control, **p < 0.01 vs. control
The relationship between mTORC1 pathway and the bone morphogenetic proteins in the radial arteries. Correlation between p-mTOR and BMP-2 expression (r = 0.762, p < 0.05), OCN expression (r = 0.815, p < 0.01) and collagen I expression (r = 0.92, p < 0.001). Correlation between p-S6K1 and BMP-2 expression (r = 0.60, p < 0.05), OCN expression (r = 0.527, p < 0.05) and collagen I expression (r = 0.701, p < 0.01)
Discussion
Chronic systemic inflammation and VC are common features in patients with ESRD. VC has been shown to be associated with an inflammatory state as well as with osteogenesis in the vascular wall [23]. Our previous study in ESRD patients demonstrated that inflammation accelerates VC by disrupting the feedback regulation of low-density lipoprotein receptor (LDLr), which is closely related to the entry of VSMCs into a transdifferentiation program of osteogenesis [24]. In this study, we investigated whether mTORC1 was involved in the progression of VC in ESRD patients under inflammatory stress.
Many inflammatory markers and mediators are found to promote VC in ESRD patients. These factors include IL-1, IL-6, CRP, TNFα and MCP-1 [25]. Hwang et al. suggested that the prevalence and extent of coronary artery calcification were greater in individuals with lower estimated glomerular filtration rate (eGFR) and higher CRP level [26]. In this study, we divided the patients into control and inflammation groups according to their plasma CRP levels. We found that the expression levels of TNF-α and MCP-1 protein were increased in the radial arteries of the inflammation group with higher plasma CRP level compared to the control group, suggesting that local arterial inflammation was consistent with a systemic inflammatory state. In addition, inflammation significantly increased calcium deposition as shown by Alizarin Red S staining, which provided clinical evidence that inflammation contributes to the progression of VC in ESRD patients.
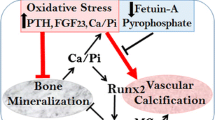
It is accepted that arterial media calcification (AMC), the main calcification mechanism in ESRD patients, is closely correlated with the conversion of VSMCs from fibroblastic to osteogenic phenotypes and the upregulation of osteogenic programs [27]. Viaene et al. reported that (micro-)inflammation plays a crucial role in the bone-vascular axis in ESRD [28]. Our previous study demonstrated that inflammation significantly increased the expression of the bone formation biomarker proteins such as BMP-2, ALP and collagen I, but decreased the expression of α-SMA in the radial arterial tissues of ESRD patients [24]. In the present study, by immunohistochemical staining, we found that the expression of BMP-2, OCN and collagen I protein were significantly upregulated in the inflammation group compared to the control group. These observations strongly suggest that chronic inflammation induces the formation of osteoblast-like cells from VSMCs in patients with ESRD. Recently, many studies have extensively explored the signaling pathways that are involved in the phenotypic change of VSMCs to osteoblast-like cells. The findings by Cheng et al. showed that peroxisome proliferator-activated receptor γ (PPARγ) regulates phosphate-induced calcification and Klotho expression in VSMCs [29]. Cai et al. suggested that Runx2 is a transcriptional target of the Wnt/β-catenin signaling pathway in VSMCs undergoing osteogenic transdifferentiation in a high-phosphate environment [13]. These results allude to the relationship between hyperphosphatemia and VC, but little is known about the mechanisms of the phenotypic conversion of VSMCs in calcified vessels in the context of an inflammatory state.
It has been reported that the mTORC1 pathway may be a link between inflammation and VC [30]. Activation of mTORC1 is dependent on inflammatory cytokines. Hu et al. found that LPS stimulation induced mTOR phosphorylation and that activation of mTOR in the epithelium was mediated by toll-like receptor 4 and a subsequent reduction in autophagy, which ultimately contributed to acute lung injury [31]. Xiao et al. demonstrated that IL-1β can activate the mTOR pathway, followed by activation of neurons, which is vital for the pathogenesis of mesial temporal lobe epilepsy [32]. Our previous in vitro studies showed that treatment with either LPS or IL-1β was sufficient to activate mTORC1 signaling in VSMCs and hepatic cells [21, 22]; moreover, in this study, we found that the expression of p-mTOR and p-S6K1 in the radial arteries was significantly elevated in the inflammation group with higher expressions of TNF-α and MCP-1, which implies that chronic inflammation could activate the mTORC1 pathway in local vasculature. Recently, the results of experiments based on mTOR inhibitors indirectly revealed that the activation of mTORC1 can contribute to the progression of osteoblastic differentiation [20, 33]. Xian et al. reported that inhibition of mTORC1 activity by rapamycin blocked insulin-like growth factor 1 (IGF-1)-induced osteoblast differentiation of mesenchymal stem cells and mineralization [33]. And Yeh et al. found that rapamycin inhibits BMP-7 induced osteogenic and lipogenic marker expressions in fetal rat calvarial cells, thereby suppressing bone formation [20]. Furthermore, Zhan et al. advanced the knowledge regarding the involvement of mTORC1 in the vasculature by showing that mTOR, through S6K1, promotes osteogenic transformation in VSMCs, possibly predisposing the vessel to calcification [34]. Indeed, our analyses in this study showed that there was a positive correlation between the activation of the mTORC1 pathway and osteogenic markers in the radial arteries. These data suggest that mTORC1 may be involved in VC by modulating the osteoblastic differentiation in calcified vessels.
In summary, our findings demonstrate that inflammation contributes to the progression of VC in ESRD via inducing osteogenic differentiation in vessels, and this is correlated with the activation of mTORC1 pathway. These observations may improve our understanding of the mechanism of VC in ESRD patients.
References
Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ (2005) Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16(2):489–495. https://doi.org/10.1681/ASN.2004030203
Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z (2008) Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces t into the uremic puzzle? Clin J Am Soc Nephrol 3(2):505–521. https://doi.org/10.2215/CJN.03670807
Kaysen GA (2001) The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol 12(7):1549–1557
Chen NC, Hsu CY, Chen CL (2017) The strategy to prevent and regress the vascular calcification in dialysis patients. Biomed Res Int 2017:9035193. https://doi.org/10.1155/2017/9035193
Yamada S, Tokumoto M, Tatsumoto N, Taniguchi M, Noguchi H, Nakano T, Masutani K, Ooboshi H, Tsuruya K, Kitazono T (2014) Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am J Physiol Renal Physiol 306(12):1418–1428. https://doi.org/10.1152/ajprenal.00633.2013
Yoshihara F (2016) Systemic inflammation is a key factor for mortality risk stratification in chronic kidney disease patients with coronary artery calcification. Circ J 80(7):1537–1538. https://doi.org/10.1253/circj.CJ-16-0506
Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E (2015) Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta 438:401–414. https://doi.org/10.1016/j.cca.2014.08.034
Lau WL, Ix JH (2013) Clinical detection, risk factors, and cardiovascular consequences of medial arterial calci cation: a pattern of vascular injury associated with aberrant mineral metabolism. Semin Nephrol 33(2):93–105. https://doi.org/10.1016/j.semnephrol.2012.12.011
Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM (2001) Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89(12):1147–1154
Neven E, Dauwe S, De Broe ME, D’Haese PC, Persy V (2007) Endochondral bone formation is involved in media calcification in rats and in men. Kidney Int 72(5):574–581. https://doi.org/10.1038/sj.ki.5002353
Towler DA (2013) Molecular and cellular aspects of calcific aortic valve disease. Circ Res 113(2):198–208. https://doi.org/10.1161/CIRCRESAHA.
Barreto FC, Barreto DV, Moyses RM, Neves CL, Jorgetti V, Draibe SA, Canziani ME, Carvalho AB (2006) Osteoporosis in hemodial-ysis patients revisited by bone histomorphometry: a new insight into an old problem. Kidney Int 69(10):1852–1857
Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J, He W (2016) WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res 345(2):206–217. https://doi.org/10.1016/j.yexcr.2016.06.007
Khosla S (2011) The bone and beyond: a shift in calcium. Nat Med 17(4):430–431. https://doi.org/10.1038/nm0411-430
Hruska KA, Mathew S, Lund RJ, Memon I, Saab G (2009) The pathogenesis of vascular calcification in the chronic kidney disease mineral bone disorder: the links between bone and the vasculature. Semin Nephrol 29(2):156–165. https://doi.org/10.1016/j.semnephrol
Alesutan I, Voelkl J, Feger M, Kratschmar DV, Castor T, Mia S, Sacherer M, Viereck R, Borst O, Leibrock C, Gawaz M, Kuro-O M, Pilz S, Tomaschitz A, Odermatt A, Pieske B, Wagner CA, Lang F (2017) Involvement of vascular aldosterone synthase in phosphate-induced osteogenic transformation of vascular smooth muscle cells. Sci Rep 7(1):2059. https://doi.org/10.1038/s41598-017-01882-2
Jewell JL, Guan KL (2013) Nutrient signaling to mTOR and cell growth. Trends Biochem Sci 38(5):233–242. https://doi.org/10.1016/j.tibs.2013.01.004
Zhao Y, Zhao MM, Cai Y, Zheng MF, Sun WL, Zhang SY, Kong W, Gu J, Wang X, Xu MJ (2015) Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via Klotho upregulation. Kidney Int 88(4):711–721. https://doi.org/10.1038/ki.2015.160
Vervloet MG, Adema AY, Larsson TE, Massy ZA (2014) The role of klotho on vascular calcification and endothelial function in chronic kidney disease. Semin Nephrol 34(6):578–585. https://doi.org/10.1016/j.semnephrol.2014.09.003
Yeh LC, Ma X, Ford JJ, Adamo ML, Lee JC (2013) Rapamycin inhibits BMP-7-induced osteogenic and lipogenic marker expressions in fetal rat calvarial cells. J Cell Biochem 114(8):1760–1771. https://doi.org/10.1002/jcb.24519
Ma KL, Liu J, Wang CX, Ni J, Zhang Y, Wu Y, Lv LL, Ruan XZ, Liu BC (2013) Activation of mTOR modulates SREBP-2 to induce foam cell formation through increased retinoblastoma protein phosphorylation. Cardiovasc Res 100(3):450–460. https://doi.org/10.1093/cvr/cvt203
Liu J, Ma KL, Zhang Y, Wu Y, Hu ZB, Lv LL, Tang RN, Liu H, Ruan XZ, Liu BC (2015) Activation of mTORC1 disrupted LDL receptor pathway: a potential new mechanism for the progression of non-alcoholic fatty liver disease. Int J Biochem Cell Biol 61:8–19. https://doi.org/10.1016/j.biocel.2015.01.011
Jung HH, Kim SW, Han H (2006) Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant 21(7):1915–1920. https://doi.org/10.1093/ndt/gfl118
Liu J, Ma KL, Gao M, Wang CX, Ni J, Zhang Y, Zhang XL, Liu H, Wang YL, Liu BC (2012) Inflammation disrupts the LDL receptor pathway and accelerates the progression of vascular calcification in ESRD patients. PLoS One 7(10):47217. https://doi.org/10.1371/journal.pone.0047217
Din ASharafE, Salem UA, Abdulazim MM DO (2016) Vascular calcification: when should we interfere in chronic kidney disease patients and how? World J Nephrol 5(5):398–417. https://doi.org/10.5527/wjn.v5.i5.398
Hwang IC, Park HE, Kim HL, Kim HM, Park JB, Yoon YE, Lee SP, Kim HK, Cho GY, Sohn DW, Kim YJ (2016) Systemic in ammation is associated with coronary artery calcifica-tion and all-cause mortality in chronic kidney disease. Circ J 80(7):1644–1652. https://doi.org/10.1253/circj.CJ-15-1224
Martínez-Moreno JM1, Muñoz-Castañeda JR, Herencia C, Oca AM, Estepa JC, Canalejo R, Rodríguez-Ortiz ME, Perez-Martinez P, Aguilera-Tejero E, Canalejo A, Rodríguez M, Almadén Y (2012) In vascular smooth muscle cells paricalcitol prevents phosphate-induced Wnt/β-catenin activation. Am J Physiol Renal Physiol 303(8):1136–1144. https://doi.org/10.1152/ajprenal.00684.2011
Viaene L, Behets GJ, Heye S, Claes K, Monbaliu D, Pirenne J, D’Haese PC, Evenepoel P (2016) Inflammation and the bone-vascular axis in end-stage renal disease. Osteoporos Int 27(2):489–497. https://doi.org/10.1007/s00198-015-3233-8.
Cheng L, Zhang L, Yang J, Hao L (2017) Activation of peroxisome proliferatoractivated receptor γ inhibits vascular calcification by upregulating Klotho. Exp Ther Med 13(2):467–474. https://doi.org/10.3892/etm.2016.3996
Montezano AC, Touyz RM (2014) Mammalian target of rapamycin: a novel pathway in vascular calcification. Can J Cardiol 30(5):482–484. https://doi.org/10.1016/j.cjca.2014.03.001
Hu Y, Lou J, Mao YY, Lai TW, Liu LY, Zhu C, Zhang C, Liu J, Li YY, Zhang F, Li W, Ying SM, Chen ZH, Shen HH (2016) Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 12(12):2286–2299. https://doi.org/10.1080/15548627.2016.1230584 doc
Xiao Z, Peng J, Gan N, Arafat A, Yin F (2016) Interleukin-1β plays a pivotal role via the PI3K/Akt/mTOR signaling pathway in the chronicity of mesial temporal lobe epilepsy. Neuroimmunomodulation 23(5–6):332–344. https://doi.org/10.1159/000460254
Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Jia X, Yakar S, Xuan S, Efstratiadis A, Wan M, Cao X (2012) Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 18(7):1095–1101. https://doi.org/10.1038/nm.2793
Zhan JK, Wang YJ, Wang Y, Wang S, Tan P, Huang W, Liu YS (2014) The mammalian target of rapamycin signalling pathway is involved in osteoblastic differentiation of vascular smooth muscle cells. Can J Cardiol 30(5):568–575. https://doi.org/10.1016/j.cjca.2013.11.005
Funding
This study was supported by grants from the National Natural Science Foundation of China (No.81500585), and Nanjing Medical Science and Technique Development Foundation (No.QRX17120).
Author information
Authors and Affiliations
Contributions
MZ and JL conceived and designed the experiments; JL performed the experiments; JL and WZ analyzed the data; CJ, YF, YX and QZ contributed reagents/materials/analysis tools; JL wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All studies were approved by the Ethical Committee of Nanjing Drum Tower Hospital. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Rights and permissions
About this article
Cite this article
Liu, J., Zhu, W., Jiang, C.M. et al. Activation of the mTORC1 pathway by inflammation contributes to vascular calcification in patients with end-stage renal disease. J Nephrol 32, 101–110 (2019). https://doi.org/10.1007/s40620-018-0486-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-018-0486-2