Abstract
Background/aims
Kidneys from uncontrolled non heart-beating donors achieve a good level of renal function after transplantation. However, a number of them will never function in the recipient. Our aim was to determine if serum biomarkers associated with platelet activity, inflammation and the nitric oxide system in uncontrolled non heart-beating donors may help to predict no renal function recovery after renal transplantation.
Methods
Serum levels of interleukin (IL)-6, IL-10, intercellular cell adhesion molecule-1 (ICAM-1), cyclic guanosine monophosphate (cGMP), nitrite + nitrate and platelet factor-4 (PF4) were measured using enzyme-linked immunosorbent assay (ELISA) kits in 88 uncontrolled non heart-beating donors divided according to the renal functionality achieved in the recipients into functional (n = 76) and non functional (n = 12).
Results
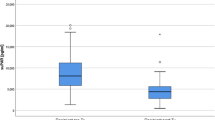
Kidneys from donors with higher IL-6 levels (>900 pg/ml) were functional after transplantation. Serum cGMP levels below 372.3 fmol/l were also associated with kidneys that recovered the renal function. However, serum levels of PF4 showed the best correlation with recovery of renal functional in the recipients since they were significantly lower in the donors whose kidneys functioned after transplantation.
Conclusions
Serum PF4 levels in uncontrolled non heart-beating donors may be a good predictor for kidneys that never will reach functional recovery. Some serum cGMP, IL-6 and IL-10 levels may simply help identify kidneys that will function after transplantation.


Similar content being viewed by others
References
Gerstenkorn C (2003) Non-heart-beating donors: renewed source of organs for renal transplantation during the twenty-first century. World J Surg 27:489–493
Kootstra G, Daemen JH, Oomen AP (1995) Categories of non-heart-beating donors. Transplant Proc 27:2893–2894
Abt PL, Desai NM, Crawford MD, Forman LM, Markmann JW, Olthoff KM, Markmann JF (2004) Survival following liver transplantation from non-heart-beating donors. Ann Surg 239:87–92
Sharif A, Borrows R (2013) Delayed graft function after kidney transplantation: the clinical perspective. Am J Kidney Dis 62:150–158
Schlumpf R, Candinas D, Weder W, Röthlin M, Zollinger A, Bleisch J, Retsch M, Largiadèr F (1993) Acute vascular rejection with hemolytic uremic syndrome in kidneys from non-heart-beating donors: associated with secondary grafts and early cyclosporine treatment? Transplant Proc 25:1518–1521
Zarifian A, Meleg-Smith S, O’donovan R, Tesi RJ, Batuman V (1999) Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int 55:2457–2466
Noris M, Remuzzi G (2010) Thrombotic microangiopathy after kidney transplantation. Am J Transplant 10:1517–1523
Sánchez-Fructuoso AI, Prats D, Torrente J, Pérez-Contín MJ, Fernández C, Alvarez J, Barrientos A (2000) Renal transplantation from non-heart beating donors: a promising alternative to enlarge the donor pool. J Am Soc Nephrol 11:350–358
Coca SG, Yalavarthy R, Concato J, Parikh CR (2008) Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 73:1008–1016
Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M (2009) Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery–a prospective cohort study. Crit Care Med 37:553–560
Aidoudi S, Bikfalvi A (2010) Interaction of PF4 (CXCL4) with the vasculature: a role in atherosclerosis and angiogenesis. Thromb Haemost 104:941–948
Yang J, Chen J, Yan J, Zhang L, Chen G, He L, Wang Y (2012) Effect of interleukin 6 deficiency on renal interstitial fibrosis. PLoS One 7:e52415
Shao J, Miyata T, Yamada K, Hanafusa N, Wada T, Gordon KL, Inagi R, Kurokawa K, Fujita T, Johnson RJ, Nangaku M (2001) Protective role of nitric oxide in a model of thrombotic microangiopathy in rats. J Am Soc Nephrol 12:2088–2097
Sacristán D, López-Farré AJ, Zamorano-León JJ, Azcona L, Fernández-Ortiz A, Romero J, Farré J, Macaya C (2008) Effects of coronary prestenting platelet inhibition on coronary poststenting inflammation. J Cardiovasc Pharmacol 51:286–292
López-Farré AJ, Modrego J, Azcona L, Guerra R, Segura A, Rodríguez P, Zamorano-León JJ, Lahera V, Macaya C (2014) Nitric oxide from mononuclear cells may be involved in platelet responsiveness to aspirin. Eur J Clin Invest 44:463–469
García-Cardoso J, Vela R, Mahillo E, Mateos-Cáceres PJ, Modrego J, Macaya C, López-Farré AJ (2010) Increased cyclic guanosine monophosphate production and endothelial nitric oxide synthase level in mononuclear cells from sildenafil citrate-treated patients with erectile dysfunction. Int J Impot Res 22:68–76
Lauzurica R, Pastor MC, Bayés B, Hernandez JM, Bonet J, Doladé M, Navarro M, Romero R (2008) Pretransplant inflammation: a risk factor for delayed graft function? J Nephrol 21:221–228
Pecoits-Filho R, Lindholm B, Axelsson J, Stenvinkel P (2003) Update on interleukin-6 and its role in chronic renal failure. Nephrol Dial Transplant 18:1042–1045
Mikłaszewska M, Korohoda P, Zachwieja K, Mroczek T, Drożdż D, Sztefko K, Moczulska A, Pietrzyk JA (2013) Serum interleukin 6 levels as an early marker of acute kidney injury on children after cardiac surgery. Adv Clin Exp Med 22:377–386
Jin Y, Liu R, Xie J, Xiong H, He JC, Chen N (2013) Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab Invest 93:801–811
Sancho A, Pastor MC, Bayés B, Sánchez A, Morales-Indiano C, Doladé M, Romero R, Lauzurica R (2010) Posttransplant inflammation associated with onset of chronic kidney disease. Transplant Proc 42:2896–2898
Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK (2003) Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol 132:24–31
Pazos P, Lima L, Casanueva FF, Diéguez C, García MC (2013) Interleukin 6 deficiency modulates the hypothalamic expression of energy balance regulating peptides during pregnancy in mice. PLoS One 8:e72339
Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, Imura H (1988) Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun 155:1459–1463
Sankaran D, Asderakis A, Ashraf S, Roberts IS, Short CD, Dyer PA, Sinnott PJ, Hutchinson IV (1999) Cytokine gene polymorphisms predict acute graft rejection following renal transplantation. Kidney Int 56:281–288
Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Fung JJ, Starzl TE (1995) Experience with liver and kidney allografts from non-heart-beating donors. Transplant Proc 27:2898
Freedman JE, Loscalzo J (2003) Nitric oxide and its relationship to thrombotic disorders. J Thromb Haemost 1:1183–1188
Sethi S, Iida S, Sigmund CD, Heistad DD (2006) Renal thrombotic microangiopathy in a genetic model of hypertension in mice. Exp Biol Med (Maywood) 231:196–203
Jakob G, Mair J, Vorderwinkler KP, Judmaier G, König P, Zwierzina H, Pichler M, Puschendorf B (1994) Clinical significance of urinary cyclic guanosine monophosphate in diagnosis of heart failure. Clin Chem 40:96–100
Mair J, Puschendorf B (1998) Is measurement of cyclic guanosine monophosphate in plasma or urine suitable for assessing in vivo nitric oxide production? Circulation 31(97):1209–1210
Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, Hoeper MM, Haller H, Fliser D (2004) Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation 20:172–177
Lew RA, Baertschi AJ (1989) Mechanisms of hypoxia-induced atrial natriuretic factor release from rat hearts. Am J Physiol 257:H147–H156
Anker SD, Coats AJ (1997) Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Circulation 95:538–539
Dussaule JC, Ardaillou R (1990) Current indications of plasma atrial natriuretic peptide measurements in human diseases. Horm Res 34:133–137
Hamet P, Pang SC, Tremblay J (1989) natriuretic factor-induced egression of cyclic guanosine 3′:5′-monophosphate in cultured vascular smooth muscle and endothelial cells. J Biol Chem 264:12364–12369
Mercapide J, Santiago E, Alberdi E, Martinez-Irujo JJ (1999) Contribution of phosphodiesterase isoenzymes and cyclic nucleotide efflux to the regulation of cyclic GMP levels in aortic smooth muscle cells. Biochem Pharmacol 58:1675–1683
Dell’anna AM, Bini Vinotti J, Beumier M, Orbegozo-Cortes D, Donatello K, Scolletta S, Vincent JL, Taccone FS (2014) C-reactive protein levels after cardiac arrest in patients treated with therapeutic hypothermia. Resucitation 85:932–938
Levine SP, Lindenfeld J, Ellis JB, Raymond NM, Krentz LS (1981) Increased plasma concentrations of platelet factor 4 in coronary artery disease: a measure of in vivo platelet activation and secretion. Circulation 64:626–632
Sachais BS, Higazi AA, Cines DB, Poncz M, Kowalska MA (2004) Interactions of platelet factor 4 with the vessel wall. Semin Thromb Hemost 30:351–358
Pitsilos S, Hunt J, Mohler ER, Prabhakar AM, Poncz M, Dawicki J, Khalapyan TZ, Wolfe ML, Fairman R, Mitchell M, Carpenter J, Golden MA, Cines DB, Sachais BS (2003) Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb Haemost 90:1112–1120
Zamani P, Schwartz GG, Olsson AG, Rifai N, Bao W, Libby P, Ganz P, Kinlay S, Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators (2013) Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the MIRACL study. J Am Heart Assoc 2:e003103
Tzoulaki I, Murray GD, Lee AJ, Rulmley A, Lowe GD, Fowkes FG (2005) C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 112:976–983
Goldberg ID, Stemerman MB, Handin RI (1980) Vascular permeation of platelet factor 4 after endothelial injury. Science 209:611–612
Acknowledgments
This work was supported by Fondo de Investigaciones de la Seguridad Social [Redes Temáticas de Investigación Cooperativa (RETICs) RD12/0042/0040], Fondo Europeo de Desarrollo Regional (Fondos FEDER). Javier Modrego is staff member of RIC, José J. Zamorano León is staff member of Comunidad de Madrid (S2010/BMD-2374). We thank Begoña Larrea for secretarial assistance.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study was approved by the local Ethical committee in accordance with the ethical guidelines of the 2000 Declaration of Helsinki and 2008 Declaration of Istanbul.
Informed consent
Written consent was obtained for all 88 renal UCNHBD.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. J. López-Farré, J. M. Santos-Sancho and J. Modrego have contributed by equal in the manuscript.
Rights and permissions
About this article
Cite this article
López-Farré, A.J., Santos-Sancho, J.M., Modrego, J. et al. Serum biomarkers in uncontrolled no heart-beating donors may identify kidneys that will never work after transplantation. J Nephrol 29, 119–127 (2016). https://doi.org/10.1007/s40620-015-0203-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0203-3