Abstract
Background and aims
Pulsed electromagnetic fields (PEMF) have proven to be an effective noninvasive method in the prevention and treatment of osteoporosis. This study evaluated the effects of PEMF on the expression of the NFATc1, CAII and RANK genes in mouse osteoclast-like cells.
Methods
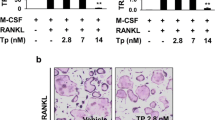
Bone marrow from bilateral tibiae and femurs was cultured in differentiation medium in the presence of soluble macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-B ligand (RANKL). After 5 days, the osteoclast-like cells were confirmed by both tartrate-resistant acid phosphatase (TRAP) staining and bone resorption assays. The osteoclast-like cells were divided into five groups and exposed to the following treatments for 3 days: M-CSF; M-CSF + RANKL; M-CSF + RANKL + osteoprotegerin (OPG), M-CSF + RANKL + premarin (E2); and M-CSF + RANKL + PEMF. The numbers of multinucleated, TRAP-positive osteoclast-like cells and resorption pits formed were determined. The expression of NFATc1, CAII and RANK mRNA was determined with real-time fluorescent quantitative polymerase chain reaction.
Results
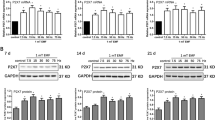
PEMF substantially reduced the number of osteoclast-like cells in the culture with M-CSF + RANKL. The level of NFATc1, CAII, and RANK mRNA expression was decreased in the M-CSF + RANKL + PEMF group compared to the M-CSF + RANKL group (p = 0.007, p = 0.039, p = 0.001, respectively). The mRNA expression of NFATc1, CAII, and RANK was not higher in the M-CSF + RANKL + OPG group compared to the M-CSF + RANKL + PEMF group (p = 0.682, p = 0.200, p = 0.924, respectively). In addition, there was no difference in the expression of mRNA from NFATc1, CAII, and RANK between the M-CSF + RANKL + PEMF group and the M-CSF + RANKL + E2 group (p = 0.853, p = 0.509, p = 0.664, respectively).
Conclusions
These data suggest that PEMF might modulate the process of osteoclastogenesis and subsequent bone resorption, at least partially, through NFATc1, CAII and RANK.


Similar content being viewed by others
References
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57. doi:10.1007/s00198-012-2074-y
Lewiecki EM (2010) Treatment of osteoporosis with denosumab. J Maturitas 66:182–186. doi:10.1016/j.maturitas.2010.02.008
Lacey DL, Timms E, Tan HL et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. J Cell 93:165–176. doi:10.1016/S0092-8674(00)81569-X
Hofbauer LC, khosla S, Dunstan CR et al (2000) The roles of osteoprotegerin:a novel secreted osteoprotegerin ligand in the regulation in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12. doi:10.1359/jbmr.2000.15.1.2
Vega D, Maalouf NM, Sakhaee K (2007) The role of receptor activator of nuclearfactor-κB (RANK)/RANK ligand/osteoprotegerin:clinical implications. J Clin Endocrinol Metabol 92:4514–4521. doi:10.1210/jc.2007-0646
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. J Ann NY Acad Sci 1092:385–396. doi:10.1196/annals.1365.035
Takayanagi H, Kim S, Koga T et al (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. J Dev Cell 3:889–901. doi:10.1016/S1534-5807(02)00369-6
Takayanagi H (2005) Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83:170–179. doi:10.1007/s00109-004-0612-6
Asagiri M, Sato K, Usami T et al (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269. doi:10.1084/jem.20051150
Zelzer E, Olsen BR (2003) The genetic basis for skeletal diseases. J Nature 23:343–348. doi:10.1038/nature01659
Mostov K, Werb Z (1997) Journey across the osteoclast. J Sci 276:219–220. doi:10.1126/science.276.5310.219
Teitelbaum SL (2000) Bone resorption by osteoclasts. J Sci 289:1504–1508. doi:10.1126/science.289.5484.1504
SatterSyed A, Islam MS, Rabbani KS, Talukder MS (1999) Pulsed electromagnetic fields for the treatment of bone fractures. J Bangladesh Med Res Counc Bull 25:6–10
Nicolakis P, Kollmitzer J, Crevenna R et al (2002) Pulsed magnetic field therapy for osteoarthritis of the knee-a double-blind sham-controlled trial. J Wien Klin Wochenschr 114:678–684
Garland DE, Adkins RH, Matsuno NN, Stewart CA (1999) The Effect of pulsed electromagnetic fields on osteoporosis at the knee in Individuals with spinal cord injury. J Spinal Cord Med 22:239–245
Jing D, Shen G, Huang J et al (2010) Circadian rhythm affects the preventive role of pulsed electromagnetic fields on ovariectomy-induced osteoporosis in rats. J Bone 46:487–495. doi:10.1016/j.bone.2009.09.021
Chen J, He H-C, Xia Q-J et al (2010) Effects of pulsed electromagnetic fields on the mRNA expression of RANK and CAII in ovariectomized Rat osteoclast-like cells. J Connective Tissue Res 51:1–7. doi:10.3109/03008200902855917
Tabrah FL, Ross P, Hoffmeier M, Gilbert FJr (1998) Clinical report on long-term bone density after short-term EMF application. J Bioelectromagnetics 19: 75–78. doi: 10.1002/(SICI)1521-186X(1998)19:2<75::AID-BEM3>3.0.CO;2-0
Tabrah F, Hoffmeier M, Gilbert F Jr, Batkin S, Bassett CA (1990) Bone density changes in osteoporosis-prone women exposed to pulsed electromagnetic fields (PEMFs). J Bone Miner Res 5:437–442. doi:10.1002/jbmr.5650050504
Pavlos NJ, Xu JK, Riedel D et al (2005) Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol J Cell Bio 125:5253–5269. doi:10.1128/MCB.25.12.5253-5269.2005
Chen J, He JQ, Zhen SY, Huang L (2012) OPG inhibits gene expression of RANK and CAII in mouse osteoclast-like cells. J Rheumatol Int 32:3393–3398. doi:10.1007/s00296-011-2338-4
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. J Methods 25:402–408. doi:10.1006/meth.2001.1262
Bell NH (2003) RANK ligand and the regulation of skeletal remodeling. J Clin Invest 111:1120–1122. doi:10.1172/JCI18358
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. J Arch Biochem Biophys 473:139–146. doi:10.1016/j.abb.2008.03.018
Grimaud E, Soubigou L, Couillaud S et al (2003) Receptor activator of nuclear factor kappa-B ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Path 163:2021–2031. doi:10.1016/S0002-9440(10)63560-2
Asagiri M, Takayanagi H (2007) The molecular understanding of osteoclast differentiation. J Bone 40:251–264. doi:10.1016/j.bone.2006.09.023
Ishida N, Hayashi K, Hoshijima M et al (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277:41147–41156. doi:10.1074/jbc.M205063200
Takayanagi H (2007) The role of NFAT in osteoclast formation. J Annals NY Acad Sci 1116:227–237. doi:10.1196/annals.1402.071
Kanis JA, Gluer CC (2001) An update on the diagnosis and assessment of osteoporosis with densitometry. J Osteoporos Int 11:192–202. doi:10.1007/s001980050281
Markov MS (2007) Pulsed electromagnetic field therapy history, state of the art and future. Environmental 27:465–475. doi:10.1007/s10669-007-9128-2
Ibiwoye MO, Powell KA, Grabiner MD (2004) Bone mass is preserved in a critical-sized osteotomy by low energy pulsed electromagnetic fields as quantitated by in vivo micro-computed tomography. J Orthop Res 22:1086–1093. doi:10.1016/j.orthres.2003.12.017
Sert C, Mustafa D, Düz MZ, Aksen F, Kaya A (2002) The preventive effect on bone loss of 50 Hz, 1 mT electromagnetic field in ovariectomized rats. J Bone Miner Metab 20:345–349. doi:10.1007/s007740200050
Chang K, Chang WH (2003) Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model:aprostagl and in E2-associated process. J Bioelectromagnet 24:189–198. doi:10.1002/bem.10078
Huang L, Wang W, Xiao D, Yang L, Lei Z, He C (2008) Effect of pulsed electromagnetic fields of different treatment time on bone mineral density of femur in ovariectomized rats. Zhong Guo Xiu Fu Chong Jian Wai Ke Za Zhi 22:548–550
Yang YH, He CQ, Yang L, Wang W, Lei ZJ (2008) Effects of different intensity pulsed electromagnetic fields on serum estradiol of ovariectomized rats. Sichuan Da Xue Xue Bao Yi Xue Ban 39:256–258
Yang L, Lei Z, He C (2006) Clinical observation of low frequency pulse electromagnetic field on treatment of osteoporosis. J Chin J Osteoporos 12:24–25. doi:10.3969/j.issn.1006-7108.2006.06.013
Luo QL, Li S-S, He C, He HC, Yang L, Deng L (2009) Pulse electromagnetic fields effects on serum E2 levels, chondrocyte apoptosis, and matrix metalloproteinase-13 expression in ovariectomized rats. J Rheumatol Int 29:927–935. doi:10.1007/s00296-008-0782-6
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81272168); the Innovation Subject of Medicine in Fujian Province (2012-CXB-32); and the Technological Project of Health Bureau, Xiamen, Fujian Province, China (3502Z20104031).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. He and Y. Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, J., Zhang, Y., Chen, J. et al. Effects of pulsed electromagnetic fields on the expression of NFATc1 and CAII in mouse osteoclast-like cells. Aging Clin Exp Res 27, 13–19 (2015). https://doi.org/10.1007/s40520-014-0239-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-014-0239-6