Abstract
Background and Objectives
Cetuximab shows activity in KRAS (Kirsten rat sarcoma viral oncogene homolog) wild-type metastatic colorectal cancer (mCRC). Recent studies have demonstrated that cetuximab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in mCRC. We investigated the associations of FcγR (fragment C γ receptor) and EGFR (epidermal growth factor receptor) polymorphisms with the outcome of mCRC patients treated with cetuximab and FOLFIRI (folic acid/5-fluorouracil/irinotecan) as second-line therapy in the FLIER (Cetuximab Plus Folinic Acid/5-Fluorouracil/Irinotecan in KRAS Wild-Type Metastatic Colorectal Cancer as a Second-Line Treatment) study.
Methods
A total of 57 patients were evaluated in this study. The association of each polymorphism with the response rate, progression-free survival, and overall survival was analyzed.
Results
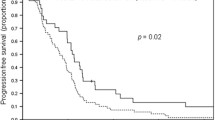
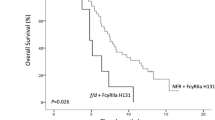
A tendency for longer overall survival was observed in patients with the EGFR CA repeat ≥36 genotype than in those with the ≤35 genotype (600 versus 483 days, P = 0.051). The haplotype containing the 131H and 158V alleles was associated with a lower response rate than the other haplotypes (P = 0.018). These results are contrary to previously published results.
Conclusion
Our data suggest that FcγR and EGFR CA repeat polymorphisms may be associated with the outcome of mCRC patients treated with cetuximab and FOLFIRI, although further investigations will be needed to confirm the association of FcγR and EGFR polymorphisms with the efficacy of cetuximab.

Similar content being viewed by others
References
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi:10.3322/caac.21149.
Tsukuma H, Ajiki W, Ioka A, Oshima A. Survival of cancer patients diagnosed between 1993 and 1996: a collaborative study of population-based cancer registries in Japan. Jpn J Clin Oncol. 2006;36(9):602–7. doi:10.1093/jjco/hyl068.
Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–14. doi:10.1016/s0140-6736(11)60613-2.
Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30(15):1755–62. doi:10.1200/jco.2011.38.0915.
Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–5. doi:10.1158/0008-5472.can-06-0191.
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65. doi:10.1056/NEJMoa0804385.
Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007;39(1–3):271–8.
van Sorge NM, van der Pol WL, van de Winkel JG. FcgammaR polymorphisms: implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61(3):189–202.
Parren PW, Warmerdam PA, Boeije LC, Arts J, Westerdaal NA, Vlug A, et al. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992;90(4):1537–46. doi:10.1172/jci116022.
Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–14.
Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist. 2007;12(9):1084–95. doi:10.1634/theoncologist.12-9-1084.
Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–7. doi:10.1200/jco.2003.05.013.
Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–96. doi:10.1200/jco.2007.14.8957.
Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–8.
Kawaguchi Y, Kono K, Mimura K, Sugai H, Akaike H, Fujii H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120(4):781–7. doi:10.1002/ijc.22370.
Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13(5):1552–61. doi:10.1158/1078-0432.ccr-06-1726.
Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27(7):1122–9. doi:10.1200/jco.2008.18.0463.
Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25(24):3712–8. doi:10.1200/jco.2006.08.8021.
Pander J, Gelderblom H, Antonini NF, Tol J, van Krieken JH, van der Straaten T, et al. Correlation of FCGR3A and EGFR germline polymorphisms with the efficacy of cetuximab in KRAS wild-type metastatic colorectal cancer. Eur J Cancer. 2010;46(10):1829–34. doi:10.1016/j.ejca.2010.03.017.
Amador ML, Oppenheimer D, Perea S, Maitra A, Cusatis G, Iacobuzio-Donahue C, et al. An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res. 2004;64(24):9139–43. doi:10.1158/0008-5472.can-04-1036.
Graziano F, Ruzzo A, Loupakis F, Canestrari E, Santini D, Catalano V, et al. Pharmacogenetic profiling for cetuximab plus irinotecan therapy in patients with refractory advanced colorectal cancer. J Clin Oncol. 2008;26(9):1427–34. doi:10.1200/jco.2007.12.4602.
Zhang W, Stoehlmacher J, Park DJ, Yang D, Borchard E, Gil J, et al. Gene polymorphisms of epidermal growth factor receptor and its downstream effector, interleukin-8, predict oxaliplatin efficacy in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2005;5(2):124–31.
Goncalves A, Esteyries S, Taylor-Smedra B, Lagarde A, Ayadi M, Monges G, et al. A polymorphism of EGFR extracellular domain is associated with progression free-survival in metastatic colorectal cancer patients receiving cetuximab-based treatment. BMC Cancer. 2008;8:169. doi:10.1186/1471-2407-8-169.
Hsieh YY, Tzeng CH, Chen MH, Chen PM, Wang WS. Epidermal growth factor receptor R521K polymorphism shows favorable outcomes in KRAS wild-type colorectal cancer patients treated with cetuximab-based chemotherapy. Cancer Sci. 2012;103(4):791–6. doi:10.1111/j.1349-7006.2012.02225.x.
Iwamoto S, Hazama S, Kato T, Miyake Y, Fukunaga M, Matsuda C, et al. Multicenter phase II study of second-line cetuximab plus folinic acid/5-fluorouracil/irinotecan (FOLFIRI) in KRAS wild-type metastatic colorectal cancer: the FLIER study. Anticancer Res. 2014;34(4):1967–73.
Okayama N, Nishioka M, Hazama S, Sakai K, Suehiro Y, Maekawa M, et al. The importance of evaluation of DNA amplificability in KRAS mutation testing with dideoxy sequencing using formalin-fixed and paraffin-embedded colorectal cancer tissues. Jpn J Clin Oncol. 2011;41(2):165–71. doi:10.1093/jjco/hyq173.
Maruta Y, Okayama N, Hiura M, Suehiro Y, Hirai H, Hinoda Y. Determination of ancestral allele for possible human cancer-associated polymorphisms. Cancer Genet Cytogenet. 2008;180(1):24–9. doi:10.1016/j.cancergencyto.2007.09.011.
Wang L, Hirayasu K, Ishizawa M, Kobayashi Y. Purification of genomic DNA from human whole blood by isopropanol-fractionation with concentrated Nal and SDS. Nucleic Acids Res. 1994;22(9):1774–5.
Rodriguez J, Zarate R, Bandres E, Boni V, Hernandez A, Sola JJ, et al. Fc gamma receptor polymorphisms as predictive markers of cetuximab efficacy in epidermal growth factor receptor downstream-mutated metastatic colorectal cancer. Eur J Cancer. 2012;48(12):1774–80. doi:10.1016/j.ejca.2012.01.007.
Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62(20):5813–7.
Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+ CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98(5):1089–99. doi:10.1002/cncr.11618.
Gebhardt F, Zanker KS, Brandt B. Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem. 1999;274(19):13176–80.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. doi:10.1056/NEJMoa033025.
Acknowledgments
This study was supported in part by a non-profit organization, the Epidemiological and Clinical Research Information Network (ECRIN), and by Japan Society for the Promotion of Science (JSPS) KAKENHI grant numbers 21591725 and 19591545. We thank Ms. Mai Hatta for her excellent clinical research coordination.
Disclosure Statement
The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the Electronic Supplementary Material.
Rights and permissions
About this article
Cite this article
Inoue, Y., Hazama, S., Iwamoto, S. et al. FcγR and EGFR Polymorphisms as Predictive Markers of Cetuximab Efficacy in Metastatic Colorectal Cancer. Mol Diagn Ther 18, 541–548 (2014). https://doi.org/10.1007/s40291-014-0103-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-014-0103-6