Abstract
Introduction
Adverse drug events (ADEs) are a significant health problem globally, with the emergency department (ED) being an important environment for the detection of ADEs. Data regarding drug-related visits to the ED in Malaysia are currently limited.
Objectives
The aim of this study was to determine factors associated with prescription drug-related ED visits at a teaching hospital.
Methods
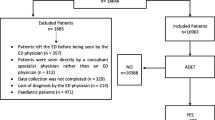
A case-control study was conducted on patients who visited the ED of Hospital Universiti Sains Malaysia over a 7-week period between December 2014 and January 2015. A visit to the ED was considered drug-related if the chief presenting complaint was related to prescription drug use. Data were collected by interviewing patients, and also from the patient’s medical record. Multiple logistic regression was applied to determine the risk factors.
Results
Overall, 144 physician-identified patients with drug-related ED visits were considered as cases and 288 patients with non-drug-related complaints were selected as controls. Independent risk factors identified for drug-related ED visits were female sex (adjusted odds ratio [OR] 1.7, 95% confidence interval [CI] 1.03–2.71), currently taking regular medication (OR 3.4, 95% CI 1.87–6.05), concurrent comorbidity (OR 2.3, 95% CI 1.28–4.10), a history of drug allergy (OR 5.36, 95% CI 2.30–12.48), and recent hospital admission (OR 2.85, 95% CI 1.23–4.10). Independent risk factors were also associated with the following health problems: diabetes mellitus (OR 6.83, 95% CI 3.30–14.12), central nervous system disorders (OR 9.42, 95% CI 3.08–14.12), and cardiovascular disorders (OR 2.5, 95% CI 1.25–4.79).
Conclusions
The determinants of a drug-related ED visit at a teaching hospital are multifactorial. Interventions to prevent future occurrences should focus on those patients at risk.
Similar content being viewed by others
References
Capuano A, Irpino A, Gallo M, et al. Regional surveillance of emergency-department visits for outpatient adverse drug events. Eur J Clin Pharmacol. 2009;65:721–8.
Wu C, Bell CM, Wodchis WP. Incidence and economic burden of adverse drug reactions among elderly patients in Ontario Emergency Departments. Drug Saf. 2012;35:769–81.
Zed PJ. Drug-related visits to the emergency department. J Pharm Pract. 2005;18:329–35.
Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ. 2008;178:1563–9.
Raschetti R, Morgutti M, Menniti-Ippolito F, et al. Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol. 1999;54:959–63.
Chen Y-C, Fan J-S, Chen M-H, et al. Risk factors associated with adverse drug events among older adults in emergency department. Eur J Int Med. 2014;25:49–55.
Feinstein JA, Feudtner C, Kempe A. Adverse drug event-related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133:e1575.
Hafner JW, Belknap SM, Squillante MD, et al. Adverse drug events in emergency department patients. Ann Emerg Med. 2002;39:258–67.
Ministry of Health, Malaysia. Annual report. Putrajaya: Ministry of Health; 2014. pp. 186–192.
Karuppannan M, Nee TK, Mohd Ali S, et al. The prevalence of adverse drug event-related admissions at a local hospital in Malaysia. Arch Pharm Pract. 2013;4:160–7.
Shehab N, Lovegrove MC, Geller AI, et al. US Emergency Department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316:2115–25.
Naing L, Than W, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch Orofacial Sci. 2006;1:9–14.
Queneau P, Bannwarth B, Carpentier F, et al. Emergency department visits caused by adverse drug events: results of a French survey. Drug Saf. 2007;30:81–8.
Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37.
Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005;5:309–16.
Tan VAK, Gerez IFA, Van Bever HP. Prevalence of drug allergy in Singaporean children. Singapore Med J. 2009;50:1158–61.
Gomes E, Cardoso MF, Praca F, et al. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34:1597–601.
Gamboa PM. The epidemiology of drug allergy-related consultations in Spanish Allergology services: Alergológica-2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):45–50.
Zopf Y, Rabe C, Neubert A, et al. Gender-based differences in drug prescription: relation to adverse drug reactions. Pharmacology. 2009;84:333–9.
Thorell K, Skoog J, Zielinski A, et al. Licit prescription drug use in a Swedish population according to age, gender and socioeconomic status after adjusting for level of multi-morbidity. BMC Public Health. 2012;12:575.
Fernandez-Liz E, Modamio P, Catalan A, et al. Identifying how age and gender influence prescription drug use in a primary health care environment in Catalonia, Spain. Br J Clin Pharmacol. 2007;65:407–17.
Al-Windi A, Elmfeldt D, Svärdsudd K. The relationship between age, gender, well-being and symptoms, and the use of pharmaceuticals, herbal medicines and self-care products in a Swedish municipality. Eur J Clin Pharmacol. 2000;56:311–7.
Kando JC, Yonkers KA, Cole JO. Gender as a risk factor for adverse events to medications. Drugs. 1995;50:1–6.
Drici M-D, Clément N. Is gender a risk factor for adverse drug reactions? Drug Saf. 2001;24:575–85.
Jayarama N, Shiju K, Prabhakar K. Adverse drug reactions in adults leading to emergency department visits. Int J Pharm Pharm Sci. 2012;4:642–6.
Maciejewski ML, Powers BJ, Sanders LL, et al. The intersection of patient complexity, prescriber continuity and acute care utilization. J Gen Intern Med. 2014;29:594–601.
Malhotra S, Karan R, Pandhi P, et al. Drug related medical emergencies in the elderly: role of adverse drug reactions and non-compliance. Postgrad Med J. 2001;77:703–7.
Voils CI, Sleath B, Maciejewski ML. Patient perspectives on having multiple versus single prescribers of chronic disease medications: results of a qualitative study in a veteran population. BMC Health Serv Res. 2014;14:490.
Hohl CM, Yu E, Hunte GS, et al. Clinical decision rules to improve the detection of adverse drug events in emergency department patients. Acad Emerg Med. 2012;19:640–9.
Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345–9.
Forster AJ, Murff HJ, Peterson JF, et al. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–23.
Kripalani S, LeFevre F, Philips CO, et al. Deficits in communication and information transfer between hospital-based and primary care physicians: Implications for patient safety and continuity of care. JAMA. 2007;297:831–41.
Coleman EA, Smith JD, Raha D, et al. Posthospital medication discrepancies: prevalence and contributing factors. Arch Int Med. 2005;165:1842–7.
Amal N, Paramesarvathy R, Tee G, et al. Prevalence of chronic illness and health seeking behaviour in Malaysian population: results from the Third National Health Morbidity Survey (NHMS III) 2006. Med J Malaysia. 2011;66:36–41.
Mafauzy M, Mokhtar N, Mohamad WW. Hypertension and associated cardiovascular risk factors in Kelantan. Med J Malaysia. 2003;58:556–64.
Sikdar KC, Alaghehbandan R, MacDonald D, et al. Adverse drug events in adult patients leading to emergency department visits. Ann Pharmacother. 2010;44:641–9.
De Paepe P, Petrovic M, Outtier L, et al. Drug interactions and adverse drug reactions in the older patients admitted to the emergency department. Acta Clin Belg. 2013;68:15–21.
Castro I, Guardiola JM, Tuneu L, et al. Drug-related visits to the emergency department in a Spanish university hospital. Int J Clin Pharm. 2013;35:727–35.
Blix HS, Viktil KK, Moger TA, et al. Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm Pract (Granada). 2010;8:50–5.
Ministry of Health, Malaysia. Annual report. Putrajaya: Ministry of Health; 2009. pp. 134.
Ministry of Health, Malaysia. Annual report. Putrajaya: Ministry of Health; 2012. pp. 175.
Acknowledgements
The authors would like to thank the physicians at the ED of the HUSM for their assistance during the study period. The assistance of nurses and other paramedics is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflicts of Interest
Ab Fatah Ab Rahman, Abubakar Ibrahim Jatau, Myat Moe Thwe Aung, and Tuan Hairulnizam Tuan Kamauzaman report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Ethical Approval
The Human Research Ethical Committee of both Universiti Sultan Zainal Abidin (UniSZA.N/1/628-1[68]) and Universiti Sains Malaysia (USM/JEPeM/140/100342) approved the study protocol. All patients selected for inclusion in the study completed consent forms prior to study commencement.
Rights and permissions
About this article
Cite this article
Ab Rahman, A., Jatau, A.I., Aung, M.M.T. et al. Factors Associated with Drug-Related Emergency Department Visits at a Teaching Hospital in Malaysia. Pharm Med 31, 175–181 (2017). https://doi.org/10.1007/s40290-017-0187-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-017-0187-5