Abstract
Background
Exercise and the subsequent recovery processes have been proposed to induce disturbances in zinc homeostasis. We previously reported acute increase in serum zinc concentration immediately after aerobic exercise; the change in the indices of zinc status during exercise recovery was not explored.
Objective
The aim of the current analysis is to determine the changes in zinc biomarkers during recovery from an aerobic exercise bout.
Methods
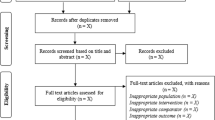
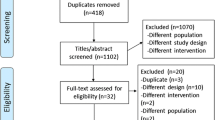
We conducted a systematic literature search on PubMed, Web of Science, Scopus and SPORTDiscus electronic databases from inception to 20 December 2014 to identify studies that investigated the acute effects of exercise on selected indices of zinc status. Meta-analyses were conducted to determine the change in serum zinc concentration during exercise recovery, defined as up to 4 h following exercise cessation, compared to pre-exercise levels.
Results
Forty-five studies were included in the systematic literature review, of which 12 studies (providing 18 comparisons) reported serum zinc levels after the cessation of exercise. During exercise recovery, serum zinc concentration was significantly lower than pre-exercise values (−1.31 ± 0.22 μmol/L, P < 0.001; mean ± SE). Secondary analyses showed a significant decrease of serum zinc levels in all categories of participants’ training status, mode of exercise and time of blood collection. Insufficient data were available for meta-analysis of other zinc biomarkers.
Conclusions
The present analysis showed that serum zinc levels decrease significantly during exercise recovery, compared to pre-exercise levels. This extends our previous report of an increase in serum zinc immediately after exercise. We postulate that the exercise-induced fluctuations in zinc homeostasis are linked to the muscle repair mechanisms following exercise; the potential for zinc to enhance the exercise recovery process remains to be determined.




Similar content being viewed by others
References
Samman S. Zinc. Nutr Diet. 2007;64:S131–4.
Fukada T, Yamasaki S, Nishida K, et al. Zinc homeostasis and signaling in health and diseases: zinc signaling. J Biol Inorg Chem. 2011;16:1123–34.
Foster M, Samman S. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal. 2010;13:1549–73.
Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–52.
Haase H, Maret W. Fluctuations of cellular, available zinc modulate insulin signaling via inhibition of protein tyrosine phosphatases. J Trace Elem Med Biol. 2005;19:37–42.
Myers SA, Nield A, Chew G-S, et al. The zinc transporter, slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PLoS One. 2013;8:e79316.
Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71.
Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–67.
Margolis LM, Pasiakos SM. Optimizing intramuscular adaptations to aerobic exercise: effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv Nutr. 2013;4:657–64.
Van Loon LJC. Is there a need for protein ingestion during exercise? Sport Med. 2014;44:S105–11.
Paulsen G, Mikkelsen UR, Raastad T, et al. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97.
Lukaski HC. Magnesium, zinc, and chromium nutriture and physical activity. Am J Clin Nutr. 2000;72:585S–93S.
Chu A, Petocz P, Samman S. Immediate effects of aerobic exercise on plasma/serum zinc levels: a meta-analysis. Med Sci Sports Exerc. 2016;48:726–33.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Academy of Nutrition and Dietetics. Evidence analysis manual: steps in the academy evidence analysis process. Chicago; 2012.
Anderson RA, Polansky MM, Bryden NA. Strenuous running—acute effects of chromium, copper, zinc and selected clinical variables in urine and serum of male runners. Biol Trace Elem Res. 1984;6:327–36.
Anderson RA, Bryden NA, Polansky MM, et al. Acute exercise effects on urinary losses and serum concentrations of copper and zinc of moderately trained and untrained men consuming a controlled diet. Analyst. 1995;120:867–70.
Arslan F. Effect of the training to the levels of the serum Zn and Cu. Asian J Chem. 2009;21:2189–92.
Bordin D, Sartorelli L, Bonanni G, et al. High intensity physical exercise induced effects on plasma levels of copper and zinc. Biol Trace Elem Res. 1993;36:129–34.
González-Haro C, Soria M, López-Colón JL, et al. Plasma trace elements levels are not altered by submaximal exercise intensities in well-trained endurance euhydrated athletes. J Trace Elem Med Biol. 2011;25(Suppl 1):S54–8.
Ohno H, Hirata F, Terayama K, et al. Effect of short physical exercise on the levels of zinc and carbonic anhydrase isoenzyme activities in human erythrocytes. Eur J Appl Physiol. 1983;51:257–68.
Ohno H, Yamashita K, Doi R, et al. Exercise-induced changes in blood zinc and related proteins in humans. J Appl Physiol. 1985;58:1453–8.
Volpe SL, Lowe NM, Woodhouse LR, et al. Effect of maximal exercise on the short-term kinetics of zinc metabolism in sedentary men. Br J Sports Med. 2007;41:156–61.
Simpson JR, Hoffman-Goetz L. Exercise, serum zinc, and interleukin-1 concentrations in man: some methodological considerations. Nutr Res. 1991;11:309–23.
van Rij A, Hall M, Dohm G, et al. Changes in zinc metabolism following exercise in human subjects. Biol Trace Elem Res. 1986;10:99–105.
Döker S, Hazar M, Uslu M, et al. Influence of training frequency on serum concentrations of some essential trace elements and electrolytes in male swimmers. Biol Trace Elem Res. 2014;158:15–21.
Khaled S, Brun JF, Cassanas G, et al. Effects of zinc supplementation on blood rheology during exercise. Clin Hemorheol Microcirc. 1999;20:1–10.
Singh A, Moses F, Smoak B, et al. Plasma zinc uptake from a supplement during submaximal running. Med Sci Sport Exerc. 1992;24:442–6.
Brown KH, Rivera JA, Bhutta Z, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:S94–203.
Chu A, Samman S. Zinc homeostasis in exercise: implications for physical performance. Vitam Miner. 2014;3:e132.
Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4:676–94.
Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev. 2000;80:681–715.
van Beaumont W, Strand JC, Petrofsky JS, et al. Changes in total plasma content of electrolytes and proteins with maximal exercise. J Appl Physiol. 1973;34:102–6.
Lowe NM, Medina MW, Stammers A-L, et al. The relationship between zinc intake and serum/plasma zinc concentration in adults: a systematic review and dose-response meta-analysis by the EURRECA network. Br J Nutr. 2012;108:1962–71.
Lukaski HC, Bolonchuk WW, Klevay LM, et al. Changes in plasma zinc content after exercise in men fed a low-zinc diet. Am J Physiol Endocrinol Metab. 1984;247:E88–93.
Lundsgaard A-M, Kiens B. Gender differences in skeletal muscle substrate metabolism—molecular mechanisms and insulin sensitivity. Front Endocrinol. 2014;5:195.
Gillum TL, Kuennen MR, Schneider S, et al. A review of sex differences in immune function after aerobic exercise. Exerc Immunol Rev. 2011;17:104–21.
Galan P, Viteri FE, Bertrais S, et al. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 2005;59:1181–90.
Gibson RS, Heath A-LM, Limbaga MLS, et al. Are changes in food consumption patterns associated with lower biochemical zinc status among women from Dunedin, New Zealand? Br J Nutr. 2007;86:71.
McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–50.
Sprouse C, Gordish-Dressman H, Orkunoglu-Suer EF, et al. SLC30A8 nonsynonymous variant is associated with recovery following exercise and skeletal muscle size and strength. Diabetes. 2014;63:363–8.
Chu A, Foster M, Ward S, et al. Zinc-induced upregulation of metallothionein (MT)-2A is predicted by gene expression of zinc transporters in healthy adults. Genes Nutr. 2015;10:44.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Anna Chu, Peter Petocz and Samir Samman declare that they have no conflicts of interest relevant to the content of this review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chu, A., Petocz, P. & Samman, S. Plasma/Serum Zinc Status During Aerobic Exercise Recovery: A Systematic Review and Meta-Analysis. Sports Med 47, 127–134 (2017). https://doi.org/10.1007/s40279-016-0567-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0567-0