Abstract
Optimizing the management of children presenting with acute severe asthma is of utmost importance to minimize hospital stays, morbidity, and mortality. Intravenous medications, including theophyllines, are used as second-line treatments for children experiencing a life-threatening exacerbation. For intravenous theophylline (aminophylline), guidelines and formularies recommend a target therapeutic range between 10 and 20 mg/l, with the commonest regimen being a loading dose of 5 mg/kg followed by an infusion calculated by age and weight. This review assesses the evidence underpinning these recommendations, highlighting the shortcomings in our understanding of the association between serum concentrations achieved, dose given, and clinical improvement experienced. To close the knowledge gap and improve outcomes for children presenting with acute severe asthma, we propose a series of research strategies to improve the assessment of illness severity, ascertain the optimal dose to maximize benefit and minimize risk, prospectively collect adverse events, and to better understand the inter-individual variation in responses to treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The aminophylline dose used for intravenous loading (5 mg/kg) in the UK and Eire does not achieve the therapeutic range expected by clinicians in most patients. |
Clinical evidence that the target therapeutic range for aminophylline of 10–20 mg/l improves meaningful clinical outcomes in severe asthma exacerbations is limited. |
Further pharmacokinetic work is needed in preparation for a clinically necessary large, three-arm, randomized controlled trial of intravenous bronchodilators in acute asthma. |
1 Background
The World Health Organization estimates that 235 million people have asthma worldwide [1]. It is the most common non-communicable disease among children. Annually, approximately 1 in 11 children in the UK receive treatment for an acute asthma exacerbation [1]. The outcomes for children are worse in the UK than for many other similar countries, with higher mortality rates. A child is admitted to hospital with acute asthma every 20 min, and 25 children died in 2015 [1]. There is an urgent clinical need to improve the morbidity and mortality associated with this condition.
In the UK, asthma is treated according to national guidelines produced by the British Thoracic Society (BTS)/Scottish Intercollegiate Guideline Network (SIGN) [2]. First-line therapy for children who present to an acute care setting with severe exacerbations involves frequent nebulized β2 agonists and ipratropium bromide driven by high-flow oxygen, plus oral steroids. In the absence of rapid improvement, or if life-threatening features are present, then second-line intravenous therapies are considered.
Three intravenous options, magnesium sulphate, β2 agonists, and aminophylline are available. The BTS/SIGN guidance does not prioritize one drug over another. UK guidance differs from international guidance. In practice, all three intravenous treatments are frequently used in the UK and Ireland. Prospective data show that, across a range of emergency departments, 3.3% (110/3238) of pediatric asthma exacerbations required intravenous therapy (alone or in combination); magnesium sulphate was used in 60.9%, salbutamol in 55.5%, and aminophylline in 47.3% of cases [3]. In total, 30 different intravenous treatment regimens were used, varying in drugs, dose, rate, and duration.
The inclusion of aminophylline as a treatment for acute severe asthma varies across the world. The Global Initiative for Asthma (GINA) guidance, used in the USA, recommends adults and children aged > 5 years receive a single bolus of magnesium sulphate only if escalating treatment is necessitated [4]. The rationale for omitting aminophylline is that a Cochrane review found it has limited efficacy and severe, potentially fatal, adverse effects. However, this review specifically excluded children from the analysis [5].
Tachycardia is a commonly reported side effect. Tachyarrhythmias have been reported in adults, but the frequency within the pediatric population is unknown. Vomiting is also a common adverse effect that can occur at all concentrations but more so at levels > 20 mg/l. Seizures have been reported in children, the mechanism and frequency of which are unknown.
Based on mechanism of action, aminophylline should be advantageous in asthma treatment. It is a methylxanthine bronchodilator composed of theophylline and ethylenediamine. Theophylline relaxes the smooth muscle of the bronchial airways and pulmonary blood vessels and reduces airway responsiveness to histamine, methacholine, adenosine, and allergen. Theophylline competitively inhibits type III and IV phosphodiesterase, the enzyme responsible for breaking down cyclic adenosine monophosphate (AMP) in smooth muscle cells, possibly resulting in bronchodilation [6]. Clinically, there is evidence that patients with life-threatening asthma may benefit from intravenous aminophylline, but the reported clinical outcomes vary [2, 7]. This may be partly attributable to not achieving the desired therapeutic range or indeed that the targeted range may not be therapeutically optimal. This review concentrates on the evidence underpinning the current dosing strategy, identifies gaps in these data, and explores how future research can help address these issues.
2 Current Dosing of Intravenous Aminophylline
Aminophylline is believed to have a narrow therapeutic range and high inter-individual variation in clearance. To avoid toxicity, UK and Eire clinicians have adopted a very consistent dosing strategy: the most common loading dose is 5 mg/kg over 20–30 min, used in > 95% of pediatric patients who received it [3]. Therapeutic drug monitoring is also commonly used, with the aim of getting concentrations within the accepted therapeutic range of 10–20 mg/l. This therapeutic range originated in the 1950s, when efficacy was only noted at a concentration > 10 mg/l in a small cohort (n = 25) of adult patients with chronic wheeze [8]. Of note, 20% of these patients had cardiac failure [8]. Increased toxicity has been seen in individual studies with adults dosed to achieve 20 mg/l compared with 10 mg/l [9]. There have been calls for alternate therapeutic ranges (e.g., 5–15 mg/l), but this has not translated into clinical practice in the UK [10].
Whether this is the optimal therapeutic range, in terms of either benefit or risk, is unclear. A recent systematic review of ten randomized controlled trials (RCTs) and two observational studies found no evidence to support this therapeutic range [11]. Children with serum levels between 10 and 20 mg/l did not show any additional efficacy (i.e., reduction in duration of symptoms, length of hospital stay, need for mechanical ventilation, or improvement in spirometry) compared with those with levels < 10 mg/l [11]. This is consistent with early studies on the effects of intravenous aminophylline, where improvements in lung function were noted across a broader therapeutic range (5–20 mg/l) [12]. The systematic review also failed to show any differences in the frequency of adverse effects in children within the therapeutic range compared with those with levels > 20 mg/l [11].
There is now significant evidence that the dose used for intravenous loading (5 mg/kg) does not achieve the therapeutic range expected by clinicians in most patients. This under-dosing was initially predicted from the 1970s pharmacokinetic (PK) work, which suggested that—in children—a dose of 5.6 mg/kg is required to achieve the target concentration of 10 mg/l [13]. Additional PK studies suggested 6 mg/kg would be a more appropriate dose to achieve 10 mg/l [14]. However, these PK studies, whilst suggesting we under-dose for the therapeutic range we are targeting, do not provide clinical teams with any feel for the likelihood of any particular serum concentration, as the inter-individual variation was so large.
Higher loading doses have been used. One RCT demonstrated the clinical benefits of aminophylline 10 mg/kg, albeit delivered over 60 min rather than 20–30 min. This study demonstrated clinical efficacy in a number of domains and achieved mean serum levels of 15.3 mg/l but also reported a significant risk of nausea and vomiting [15].
This highlights another dosing uncertainty: for how long to infuse the medication, as this will affect the PK of the drug. A recent systematic review of the loading dose found a poor relationship between administered dose and symptom resolution [16].
These uncertainties in dosing have not been addressed using physiologically based PK modelling (PBPK), where vast in silico populations of children can be modelled to provide population estimates of PK. For intravenous aminophylline, this has only been undertaken for the current loading dose of 5 mg/kg infused over 20 min. The study found it would produce a serum concentration of < 10 mg/l in 70.3% of children, 10–20 mg/ml in 29.4%, and > 20 mg/l in only 0.1% of children who receive it [17]. This was corroborated by clinical data collected in the same study [17]. However, almost all children would achieve a serum concentration of 5–15 mg/l using this loading dose (Fig. 1). Is the therapeutic range sub-optimal, the dose incorrect, or both? How does this compare with the 1-h duration 10 mg/kg loading dose?

(Adapted from Cooney et al. [17])
Serum concentration time profile for theophylline in children aged 1 month–18 years modelled using physiologically based pharmacokinetic modelling (PBPK) software. Black line is mean profile, grey lines are 5th and 95th percentiles, and open circles are clinical data. More than 95% achieve a serum concentration of 5–15 mg/l (green lines) using the current loading dose of aminophylline 5 mg/kg; however, the current recommended therapeutic range is 10–20 mg/l.
Following the loading dose, an aminophylline infusion may be used to maintain serum concentrations in the therapeutic range. There is even less data supporting the optimal dose (rate) of this infusion than there is for the loading dose. The recommended dose in the British National Formulary for Children (BNFC) for intravenous aminophylline infusion is based on age, with those aged < 11 years receiving 1 mg/kg/h and those aged 12–18 years receiving 0.5–0.7 mg/kg/h [18], with both rates titrated to achieve the therapeutic range (10–20 mg/l). The PK modelling work suggests that, for younger children (aged 0–11 years) receiving 1 mg/kg/h initially, the mean steady state would be 16.4 mg/l (95th centile range 5.3–32 mg/l). Older children (aged 12–18 years) receiving the 0.5 mg/kg/h infusion would achieve a mean concentration of 9.4 mg/l (3.4–18 mg/l), whereas the higher dose would achieve 13.3 mg/l (4.7–25.0 mg/l). Clearly, a wide range of possible outcomes related to the high inter-individual variation is seen with this medication, and many children will be over- or under-dosed. Table 1 shows the percentage of children with very low (< 5 mg/l), sub-therapeutic (5–10 mg/l), therapeutic (10–20 mg/l), and supra-therapeutic (> 20 mg/l) serum concentrations.
3 Future Research
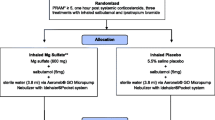
It is unacceptable that more than 20 children died from acute asthma attacks in the UK in 2015. While some of the deaths will have occurred before arrival at hospital, this should still serve as a stimulus to improve the treatment and outcomes for children with acute severe asthma in hospital. Several interconnected research strategies framed around the understanding of the disease, the classification of illness severity, the medications used, and the inter-individual variation present are required (Fig. 2).
Before any drug studies are carried out, the optimal method of collecting illness severity scores in childhood asthma must be determined and implemented nationally. In the UK, emergency asthma admission rates vary 25-fold [19]. Local authorities in the north-west of England include seven of the ten highest rates of emergency admissions for childhood asthma in the UK [20], but the mortality in this region is the same as the national average. Are the admitted children in these areas more unwell? Do they have less access to preventive healthcare or more environmental triggers? Or do the medical teams have lower thresholds for admission? Routine collection of validated pediatric asthma severity scores in pediatric emergency departments would both help answer these questions and streamline the implementation of asthma clinical trials into hospitals nationally.
In parallel with this, observational PK studies linking aminophylline serum concentrations with clinically important outcomes need to be designed. This should be done in parallel with patients and parents and use the core outcome set for pediatric asthma to guide the outcomes selected [21]. For most dosing research, a new drug with unknown PK is tested at a range of doses to look for alterations in disease biomarkers. However, we have an old drug used in emergency situations at a standardized dose, which is acceptable to UK clinicians, who are aware that, at high concentrations, significant adverse effects can occur. The evidence to date on intravenous aminophylline may not support the dose and therapeutic range as being optimal. It is therefore unlikely that, in the extremely serious clinical scenario of an acutely unwell child with asthma who requires second-line intravenous treatment, clinicians would accept randomization to other doses that may decrease efficacy or increase adverse effects. It may therefore be necessary to undertake observational dosing research using the existing clinical intravenous aminophylline dose.
The PBPK work has shown considerable inter-individual variation in the concentrations achieved using the intravenous aminophylline dose common in routine clinical practice. Prospective collection of data from children aligned with accurately timed therapeutic drug monitoring sampling could provide a range of concentrations that could be linked to outcomes. If differences exist in outcomes across the various serum concentrations achieved, then dose modification to maximize the proportion of children who achieve that concentration will need to be undertaken. The PBPK models already exist, so this would be relatively quick to undertake and put into practice.
As well as establishing the PK-pharmacodynamic (PD) relationship for efficacy, we need to consider safety. The prospective collection of adverse effect data would need to be integral to an observational dosing study, and the optimal dose will likely be a balance between efficacy and adverse effects. Even if the optimal benefit: risk ratio occurs at a serum concentration consistent with the current dosing, these data will provide assurance that current dosing strategies are appropriate.
Similarly, the serum concentrations targeted with the intravenous aminophylline infusion need to be examined. This will be additionally complicated by the titration of doses to meet the current therapeutic range. Careful consideration will be needed to decide whether to work within the current dosing methodologies and extract the most useful information to guide future treatments or establish whether clinical teams would be willing to undertake randomized trials in this area.
There may also be a place for stratified medicine in the dosing of aminophylline, particularly during the infusion, to guide rate. Theophylline is predominantly metabolized by cytochrome P450 (CYP)-1A2 [22], which may have polymorphisms that affect clinical outcomes. The effects of the CYP1A2 polymorphisms on theophylline metabolism are poorly understood. Studies investigating this relationship are inconsistent, with some studies demonstrating an effect of the *1F/*1F polymorphism on expression and higher inducibility of CYP1A2 [23, 24]. Including pharmacogenomic analysis with the collection of the PK data would therefore help answer this and other questions and, with the advent of bedside genetic testing for specific polymorphisms, a path to implementation can be envisioned.
Even if an optimal dose of intravenous aminophylline is established, we are still unclear on which of the three intravenous treatments (aminophylline, salbutamol, and magnesium sulphate) for acute severe asthma in children is optimal. There is a case for an RCT of the three intravenous treatments. One such study has already been undertaken, although there are concerns of excessive heterogeneity in the study group. The sample size was small (n = 100), and the age range of the participants includes very young children, down to the age of 1 year, lower than UK pediatricians would reliably diagnose with asthma. In addition, the choice of β2 agonist (terbutaline) and the doses of magnesium and terbutaline used are not consistent with current UK practice [25].
The lack of evidence for aminophylline, as well as for the intravenous salbutamol dose and expected effect sizes for all three intravenous treatment options means a definitive RCT in the UK is some way from reality [3, 26, 27]. There is some progress, such as a recent PK study of salbutamol in pediatric intensive care unit (PICU) patients [28], but too many gaps in the evidence remain. Even if the evidence supporting the dose of aminophylline, salbutamol, and magnesium sulphate were sufficient, this RCT would be a complex and expensive study, possibly prohibitively so, requiring multiple sites recruiting children in extremis, likely using a deferred consent model. Sites around the country will have their own standard of care and local guidelines that specify the order in which the intravenous treatments are given and therefore may not be in equipoise. Would cluster randomization of centers be an acceptable way forward? How does the current variation in admissions for acute presentation affect the choice of sites and drugs used? What about clinicians who are not in equipoise? Clearly, expert clinical trial design and statistical input would be required to tackle these confounders.
It is possible to view this as too complicated or too far from current implementation to consider, but for the children and young people affected, it is a question of vital importance. While any grand RCT remains out of reach, other initiatives are being picked up to help answer the important questions about intravenous aminophylline. We remain hopeful that, as each question is answered, the goal of evidence-based intravenous treatments for acute severe asthma moves closer.
4 Conclusion
The management of acute severe asthma includes intravenous aminophylline, but—in common with the other intravenous treatments available—evidence supporting the current dosing regimen is sparse. Prospective studies collecting pre-determined core outcomes, validated severity scores, PK parameters, adverse effects, and genomic data are needed to optimize the treatment of children with acute severe asthma using intravenous aminophylline.
References
The Asthma UK Data Portal 2017. http://www.asthma.org.uk/get-involved/campaigns/data-portal/. Accessed 18 Aug 2017.
James DR, Lyttle MD. British guideline on the management of asthma: SIGN Clinical Guideline 141, 2014. Arch Dis Child Educ Pract Ed. 2016;101(6):319–22.
Morris I, Lyttle MD, O’Sullivan R, Sargant N, Doull IJ, Powell CV, et al. Which intravenous bronchodilators are being administered to children presenting with acute severe wheeze in the UK and Ireland? Thorax. 2015;70(1):88–91.
Global Initiative for Asthma (GINA) 2017. http://ginasthma.org/. Accessed 18 Aug 2017.
Nair P, Milan SJ, Rowe BH. Addition of intravenous aminophylline to inhaled beta2-agonists in adults with acute asthma. The Cochrane Library. 2012.
Drugbank Drug created on June 13, 2005 07:24. https://www.drugbank.ca/drugs/DB00277. Accessed 11 June 2017.
Neame M, Aragon O, Fernandes RM, Sinha I. Salbutamol or aminophylline for acute severe asthma: how to choose which one, when and why? Arch Dis Childhood Educ Pract Ed. 2015;100(4):215–22.
Turner-Warwick M. Study of theophylline plasma levels after oral administration of new theophylline compounds. Br Med J. 1957;2(5036):67–9.
Holford N, Black P, Couch R, Kennedy J, Briant R. Theophylline target concentration in severe airways obstruction—10 or 20 mg/L? Clin Pharmacokinet. 1993;25(6):495–505.
Self TH, Heilker GM, Alloway RR, Kelso TM, Aboushala N. Reassessing the therapeutic range for theophylline on laboratory report forms: the importance of 5–15 μg/ml. Pharmacotherapy. 1993;13(6):590–4.
Cooney L, Hawcutt D, Sinha I. The evidence for intravenous theophylline levels between 10-20 mg/L in children suffering an acute exacerbation of asthma: a systematic review. PLoS One. 2016;11(4):e0153877.
Mitenko PA, Ogilvie RI. Rational intravenous doses of theophylline. N Engl J Med. 1973;289(12):600–3.
Loughnan P, Sitar D, Ogilvie R, Eisen A, Fox Z, Neims A. Pharmacokinetic analysis of the disposition of intravenous theophylline in young children. J Pediatr. 1976;88(5):874–9.
Hendeles L, Weinberger M, Bighley L. Disposition of theophylline after a single intravenous infusion of aminophylline 1–4. Am Rev Respir Dis. 1978;118(1):97–103.
Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child. 1998;79(5):405–10.
Cooney L, Sinha I, Hawcutt D. Aminophylline dosage in asthma exacerbations in children: a systematic review. PLoS One. 2016;11(8):e0159965.
Cooney L, McBride A, Lilley A, Sinha I, Johnson TN, Hawcutt DB. Using pharmacokinetic modelling to improve prescribing practices of intravenous aminophylline in childhood asthma exacerbations. Pulm Pharmacol Ther. 2017;43:6–11.
Paediatric Formulary Committee. British National Formulary for Children. London: BMJ Group, Pharmaceutical Press. London: RCPCH Publications; 2016.
Chief Medical Officer. Annual Report of the Chief Medical Officer: Our Children Deserve Better: Prevention Pays 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/255237/2901304_CMO_complete_low_res_accessible.pdf. Accessed 18 Aug 2017.
National Child and Maternal Health Intelligence Network. Hospital admissions for asthma Public Health England. 2014. http://atlas.chimat.org.uk/IAS/dataviews/report/fullpage?viewId=365&reportId=327&geoId=4&geoReportId=4204. Accessed 18 Aug 2017.
Sinha IP, Gallagher R, Williamson PR, Smyth RL. Development of a core outcome set for clinical trials in childhood asthma: a survey of clinicians, parents, and young people. Trials. 2012;13(1):103.
Ha HR, Chen J, Freiburghaus AU, Follath F. Metabolism of theophylline by cDNA-expressed human cytochromes P-450. Br J Clin Pharmacol. 1995;39(3):321–6.
Obase Y, Shimoda T, Kawano T, Saeki S, Sy Tomari, Mitsuta-Izaki K, et al. Polymorphisms in the CYP1A2 gene and theophylline metabolism in patients with asthma. Clin Pharmacol Ther. 2003;73(5):468–74.
Aklillu E, Carrillo JA, Makonnen E, Hellman K, Pitarque M, Bertilsson L, et al. Genetic polymorphism of CYP1A2 in Ethiopians affecting induction and expression: characterization of novel haplotypes with single-nucleotide polymorphisms in intron 1. Mol Pharmacol. 2003;64(3):659–69.
Singhi S, Grover S, Bansal A, Chopra K. Randomised comparison of intravenous magnesium sulphate, terbutaline and aminophylline for children with acute severe asthma. Acta Paediatr. 2014;103(12):1301–6.
Hawcutt DB, Cooney L, Oni L, Pirmohamed M. Precision dosing in children. Exp Rev Precis Med Drug Dev. 2016;1(1):69–78.
Starkey ES, Mulla H, Sammons HM, Pandya HC. Intravenous salbutamol for childhood asthma: evidence-based medicine? Arch Dis Child. 2014;99(9):873–7.
Vet NJ, de Winter BC, de Wildt SN, der Nagel BC, Knibbe CA, Koninckx M, et al. Population pharmacokinetics of intravenous albuterol in children with status asthmaticus. Arch Dis Childhood. 2016;101(1):e1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors, Dr. Gemma L. Saint, Dr. Malcolm G. Semple, Dr. Ian Sinha, and Dr. Daniel B. Hawcutt have no conflicts of interest.
Funding
No funding was received to assist with the preparation of this manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saint, G.L., Semple, M.G., Sinha, I. et al. Optimizing the Dosing of Intravenous Theophylline in Acute Severe Asthma in Children. Pediatr Drugs 20, 209–214 (2018). https://doi.org/10.1007/s40272-017-0281-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-017-0281-x