Abstract
Background
Patients with end-stage renal disease (ESRD) require phosphate binders for hyperphosphatemia and erythropoiesis-stimulating agents (ESAs) and intravenous (IV) iron for anemia. Ferric citrate (FC) is a novel, iron-based phosphate binder that increases iron stores and decreases IV iron and ESA usage while maintaining hemoglobin levels, and may decrease the cost of ESRD care. The study objectives were to (1) quantify differences in ESA and IV iron usage among ESRD patients receiving FC compared with active control (AC) (sevelamer carbonate and/or calcium acetate) on the basis of data from a 52-week phase III clinical trial and (2) standardize trial data to the general United States (US) ESRD population and calculate the potential impact of FC on ESRD cost/patient/year in the USA.
Study Design
The study was a randomized, controlled clinical trial.
Setting and Population
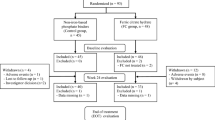
A total of 441 adult subjects with ESRD who received FC or AC for 52 weeks were included.
Model, Perspective, and Timeline
Differences in ESA and IV iron usage between the treatment groups were modeled over time using generalized linear mixed models and zero-inflated Poisson models. Trends were modeled via logarithmic curves, and utilization patterns were applied to the general dialysis population to estimate expected resource savings.
Outcomes
Study outcomes were costs saved/patient/year using FC versus AC (US dollars).
Results
Our model suggests an annual decrease of 129,106 U of ESAs and 1960 mg of IV iron per patient in the second year after a switch from AC to FC. Applying 2013 Medicare pricing, this would save $1585 in ESAs and $516 in IV iron: a total of $2101/patient/year; these savings would be expected to double for managed care plans.
Limitations
The projections were made on 1 year of trial data.
Conclusions
Phosphate binding with FC reduces IV iron and ESA usage. Given the high cost burden of ESRD, our model demonstrates significant potential cost savings.
Trial Registration
ClinicalTrials.gov (NCT01191255) http://clinicaltrials.gov/ct2/show/NCT01191255.
Similar content being viewed by others
Hyperphosphatemia and anemia are nearly universal in patients with end-stage renal disease (ESRD), and are one of the more costly aspects of ESRD-related care. |
In a phase III, 52-week clinical trial in ESRD study subjects on dialysis, ferric citrate, an FDA-approved iron-based phosphate binder, significantly reduced erythropoiesis-stimulating agent (ESA) and intravenous (IV) iron use when compared with study subjects receiving a non-iron-based phosphate binder (“active control”). |
The costs savings we predict in our report are from analyses of the IV iron and ESA usage from that phase III trial over the entire 52-week active-control study period. |
The percentage of subjects on ferric citrate receiving IV iron decreased, declining from nearly 60 % at the beginning of the study to approximately 20 % by the end of the study. |
In fourth-quarter 2013 Medicare pricing terms, these differences would equate to $1585 in ESAs and $516 in IV iron, for a total saving of $2101/patient/year for dialysis centers, and twice that, $4202/patient/year, for managed care plans. |
1 Introduction
Dietary modifications and treatment with dialysis are usually ineffective strategies to control serum phosphorus to target levels in patients with end-stage renal disease (ESRD). Oral phosphate binders, therefore, become an important adjunct in the care of the majority of these patients [1]. Oral phosphate binders include calcium carbonate, calcium acetate, sevelamer, lanthanum carbonate, and aluminum hydroxide. While each of these is effective in binding dietary phosphorus in the gastrointestinal tract, they have a number of differences in tolerability and potential side effects [2–4].
Ferric citrate (FC) is a novel, iron-based, oral phosphate binder that has been shown in clinical trials to provide safe and effective management of serum phosphorus in subjects with ESRD [5–10]. Similar to other binders, FC binds dietary phosphorus in the bowel lumen; the insoluble ferric phosphate product then precipitates and is subsequently excreted. Although formal pharmacokinetic studies have not been performed, examination of serum iron parameters has shown that there is also systemic absorption of iron from FC [11].
Like hyperphosphatemia, anemia is nearly universal in patients with ESRD and is one of the most costly aspects of ESRD-related care [12]. Erythropoiesis-stimulating agent (ESA) dose requirements are less when patients have adequate levels of circulating iron; thus, intravenous (IV) iron is commonly administered to patients receiving ESAs [13–15].
Primary analysis of the 52-week active-control (AC) period of the phase III study demonstrated that the use of FC significantly increased serum ferritin and transferrin saturation compared with AC, and reduced IV iron and ESA requirements in subjects receiving FC while maintaining hemoglobin levels [16, 17]. Given the costs of managing anemia, the use of FC as a phosphate binder for patients with ESRD may also have an impact on the cost of caring for patients with ESRD. This report presents models to determine the potential cost savings associated with the reduced ESA and IV iron utilization seen in the pivotal phase III trial of FC compared with AC.
2 Materials and Methods
2.1 Clinical Trial
Data for these analyses were drawn from the 52-week AC period of a phase III, international, multicenter, AC and placebo-controlled, randomized, open-label trial of the efficacy and safety of FC as a treatment for hyperphosphatemia in subjects with ESRD (NCT01191255) [8, 16]. The institutional review board at the Clinical Coordinating Center at Vanderbilt University and each clinical site approved the initial trial from which the data for this study was obtained. All subjects gave written informed consent before any investigational procedures, and the trial was conducted in accordance with the Declaration of Helsinki and good clinical practice.
After a 2-week washout period from all phosphate-binding agents, subjects were randomly assigned in a 2:1 ratio to receive FC or AC, which consisted of study-supplied calcium acetate and/or sevelamer carbonate. A total of 441 subjects at 60 study sites in the USA and Israel were randomized. During the 52-week AC period, binder dosing was titrated to a goal serum phosphorus level between 3.5 and 5.5 mg/dL.
2.2 Calculation of Utilization
Intravenous iron and ESA usage in each treatment arm were calculated by dividing the 52-week AC period into thirteen 28-day periods. This provided a more uniform distribution of dosing periods, allowing us to match trial data with the weekly totals reported by the United States Renal Data System (USRDS) for 2011 [18]. Case report forms (CRFs) from study visits were used to define treatment episodes (start date to stop date on the CRF) for each medication. Average daily doses for each treatment episode were calculated and then divided among specific 28-day periods in the study. A weighted average for each 28-day period was calculated to allow direct comparisons of dose. For their data to qualify for inclusion in a given 28-day period, the subject was required to have spent at least 15 days of the period as active in the study and on assigned therapy. Total dose for subject-months, with 15–27 days of included data, was rescaled to the expected value over 28 days. Darbepoetin units were converted to epoetin alfa-equivalent units (200 U per microgram of darbepoetin) [8, 16]. Subjects contributed time and data for only those periods in which they were active in the study and receiving their randomly assigned phosphate binder treatment.
2.3 Reporting Data and Statistical Significance
Differences between the FC and AC arms in ESA and IV iron dose were calculated for each 4-week period for three values: percentage of subjects receiving the drug, mean and standard deviation of dose among those receiving the drug, and mean and standard deviation of overall utilization across all subjects (including those with 0 dose). Statistical analysis of percentage data was conducted using generalized linear mixed models, which allow for logistic modeling of non-normal data with correlations [19]. These data were zero-inflated; a significant proportion of subjects (>15 %) had no administered doses in a given 4-week period. For this reason, overall utilization was considered to be the most useful continuous comparator. The significance of differences between the groups was modeled using a zero-inflated Poisson (ZIP) model with degrees of freedom and standard errors adjusted using the Kenward–Roger method [18]. Doses over days 1–28 of the study were used for each subject as their starting value, and significance was assessed in the following 4-week periods. Demographic comparisons between treatment groups were made using unpaired t tests for continuous variables, such as weight and age, and Chi-square tests for categorical variables, such as race and gender.
2.4 Estimates of Economic Impact
Percentage differences between the treatment arms were mapped to USRDS data for ESA and IV iron use from 2011 (reference population) [18], the latest available and a match for the time frame of this 2010–2012 study. Any differences in utilization between the FC and AC arms were standardized to the 2011 USRDS reference population. The time periods of the study and the USRDS data do not precisely match because of variability in enrollment dates for the study (starting in December 2010), but the overall temporal trend was considered to be relevant.
Since the changes in IV iron and ESA usage over time were nonlinear in the phase III trial and the USRDS data, we utilized logarithmic regression analysis to create curves to estimate usage patterns over time, modeling mean dose for those receiving the drug, percentage of subjects receiving the drug, and mean utilization for all subjects. Values for the treatment groups were projected beyond the trial period under two different assumptions: the achievement of a steady-state and a continued change along the same curve. Estimates of differences in ESA and IV iron utilization between the groups for 1 or 2 years were generated on the basis of the assumption that all patients remain on therapy for the duration of that time period. Thus, variability (e.g., standard deviation) was not directly calculable for these economic estimates.
For a general estimate of the economic impact of differences in medication utilization among Medicare patients, we applied fourth quarter (Q4) 2013 Medicare average sale prices (ASPs) plus price changes equal to those seen in published ASPs in 2013 (+9.4 % for ESA; −3.2 % for IV iron sucrose) [20, 21]. In the case of Medicare, under the current bundle system, these savings would be realized by dialysis providers. We conservatively estimated the economic impact on commercial payers by doubling these figures [22, 23]. We modeled annual per-patient utilization and cost differences for the second year (and projected following years) after a theoretical switch from AC to FC for both Medicare and commercial pay patients. All costs and savings are presented in US dollars.
3 Results
3.1 Demographics
Among the 438 subjects in this population (those among the 441 randomized subjects who received at least one dose of study drug), no notable differences were found with regard to age, sex, weight, race, or ethnicity in the AC versus FC groups (Table 1). Of the 289 subjects assigned to FC treatment, 277 (95.8 %) completed at least 15 days of a 4-week period and had valid data on injectable medications. In the AC group, 145 (97.3 %) of 149 met these requirements.
3.2 Erythropoiesis-Stimulating Agent Utilization
Both the percentage of subjects receiving ESAs and the mean dose for those subjects were similar in the first 4-week period of the study (Table 2). During the remainder of the 52 weeks, a gap in ESA utilization between the two treatment groups emerged due to differences in both the relative percentage of subjects receiving ESAs and the mean dose administered over the period among those receiving an ESA, primarily the latter.
In the FC group, mean dose for subjects receiving ESAs in a given 4-week period declined more precipitously than in the AC group. The differences in overall per-subject ESA use between the FC and AC groups reported in Table 2 take into account differences in both doses and percentage use and provide the best comparison of utilization. These reductions ranged from 5000 to 10,000 U per 4-week period over the course of the study. ZIP models indicated that between-group differences were highly significant (P < 0.001) at all time points assessed (periods 2–13). Total per-subject ESA use was 74,194 U lower in the FC group compared with the AC group across the 52 weeks of the trial.
Temporal trends for both treatment groups and USRDS data, estimated by logistic regression, are presented in Fig. 1 along with estimates of reductions in ESA utilization that might be expected in subjects who switch from current phosphate binders to FC. Throughout the study, mean doses for subjects receiving ESAs were substantially lower in the study-eligible population than those reported by the USRDS. When standardized to the USRDS reference population, the trial-based savings of 74,194 U per subject over the first year rose to 97,824 U per subject.
Based on the regression model, the reduction in USRDS-adjusted utilization would project to 140,533 U per patient in the second year of FC therapy (Fig. 1). Alternatively, if at the end of the trial period a steady-state difference between the groups was assumed, the total reduction in year 2 was expected to be 129,106 U per subject. Based on Medicare pricing at the end of 2013, the adjusted cost difference for the steady-state estimate would be $1585/patient/year.
3.3 Intravenous Iron Utilization
The percentage of subjects receiving IV iron and the mean dose received were similar between the two treatment groups in the first 4-week period (Table 3). As with ESAs, a gap in IV iron utilization between the two treatment groups emerged during the remainder of the 52 weeks of the study. In this case, these differences were primarily due to the relative percentage of subjects in each group receiving IV iron. In the FC group, the percentage of subjects receiving IV iron in a given 4-week period declined steadily over the course of the study, falling from 58.8 % in the first period to between 19.3 and 22.7 % per 28 days over the last 20 weeks of the 52-week period.
Differences in the percentage of subjects receiving IV iron between the two groups were statistically significant starting in month 3 and continuing through to the end of the study (P < 0.05 for month 3 and P < 0.01 for months 4–13). The mean dose administered to subjects receiving IV iron was also lower in the FC group over the period. Differences in overall utilization between the FC and AC groups, taking into account reduced percentages and differences in mean dose, are presented in Table 3. According to the ZIP model, total utilization was significantly different between the groups in all but two 4-week periods (8 and 11). The mean total per-subject IV iron utilization was 677.1 mg lower in the FC than in the AC group across the 52-week AC period of the trial.
Temporal trends in total utilization for both treatment groups and the USRDS population were modeled by logistic regression. These models are presented in Fig. 2 along with estimates of reductions in IV iron use that might be expected in patients who switch from current phosphate binders to FC. When standardized to the reference USRDS population, the trial-based savings of 677.1 mg per subject over the year rises to 1407.7 mg per subject.
If the differences between treatment groups were to continue to widen in year 2 after initiation of FC (as in Fig. 2), the reduction in USRDS-standardized utilization would increase to 2267.8 mg per patient. If a steady-state difference between the groups was assumed at the end of the trial period, the total reduction during year 2 was expected to be 1960.6 mg. Based on Medicare pricing at the end of 2013, the adjusted cost difference under this steady-state estimate would be $516/patient/year.
4 Discussion
This analysis details substantially reduced utilization of anemia-related medications among subjects receiving FC compared with AC. These findings, when applied to the general US dialysis population, translate into significant health care dollar savings.
At the end of the 52-week, AC period of the trial, the FC-treated group was, on average, receiving 20–25 % less ESA per subject than the AC-treated group. Reductions in ESA use over time were also found in the AC group, but these reductions were far less substantial and in line with real-world trends documented in the USRDS data. Between-group differences were highly significant (P < 0.001) at all time points assessed.
The percentage of FC-treated subjects receiving IV iron in a given 4-week period declined from nearly 60 % to approximately 20 % by the end of the study. Over the last 40 weeks, total IV iron usage in the FC group was less that 50 % of that in the AC group. Month-by-month differences were statistically significant (P < 0.05) for all but two periods (8 and 11). This inconsistency may be due to bolus dosing of iron and the instability of short-term averages in a relatively small pool (fewer than 40 subjects in each group were receiving IV iron by the end of the study).
Logistic regression models indicated that these differences in overall utilization continue into the second year of FC treatment. These differences remained even under a steady-state assumption, suggesting an annual saving of 129,106 U of ESA (as epoetin alfa equivalents) and 1960.6 mg of IV iron per patient in the second year and onwards.
The ESA and IV iron sparing effect of FC should impact public and private payers as well as dialysis clinics. Although federal law allows for Medicare coverage of ESRD treatment, private insurance remains the primary payer for the first 30 months of dialysis for patients who have it in place [23]. Thus, it is appropriate to estimate savings for both the Medicare-covered patient as well as those covered by private insurance or some other means.
Reduced use ESA and IV iron among Medicare patients would primarily benefit dialysis providers, as these expenses are included in the ESRD Prospective Payment System (the “Bundle”) [18]. Reduced use among patients with managed care plans would primarily benefit the plans themselves while slightly reducing profits for dialysis providers. Commercial payers reimburse dialysis centers directly for IV medication use and do so at a considerably higher rate than the price of the drugs, according to published Medicare-based figures. The largest dialysis organizations (DaVita and Fresenius) both report that the commercial reimbursement rate for them is at least two times greater than the Medicare ASP for injectable medications administered at the dialysis center [22, 23].
In Q4 2013 Medicare pricing terms, these differences would equate to $1585 in ESAs and $516 in IV iron, for a total saving of $2101/patient/year for dialysis centers, and twice that, $4202/patient/year, for managed care plans.
For these calculations and projections, the patient population was limited to those who were active in the study and receiving their initially assigned phosphate binder. The decline in the percentage of subjects receiving ESAs in the AC group matched trends in 2011, according to USRDS data, although a slightly lower percentage of subjects in the AC group received ESAs in any given period (75.0–84.3 %) than would be expected from USRDS data (80.1–84.3 %) [18]. Throughout the study, the percentage of subjects receiving IV iron in the AC group was lower than that in the 2011 USRDS population. Doses received for subjects taking IV iron did not show a consistent pattern, but were generally lower in the AC group than in USRDS data.
Reductions in utilization were applied to a roughly contemporary reference population based on USRDS data [18]. The subjects in the study were generally representative of the US dialysis population as evidenced by the prevalence of diabetes and cardiovascular disease in the trial cohort [16]. However, as is the case in all study populations, there were also some differences. The mean age in the USRDS 2011 reference population was 63 for hemodialysis patients versus 55 in the study population, and the USRDS percentage of subjects aged 65 years and older was 43.9 % versus 20.5 % in the study. The gender breakdown, however, was similar; 57 % of reference USRDS hemodialysis patients were male, while 58 % were male in the study [18]. It was also codified in the entry criteria that subjects must have an estimated >1-year life expectancy as determined by each site’s primary investigation (PI). It is possible that there will be differences in the impact of FC on ESA and IV iron usage in the wider dialysis population. The USRDS-adjusted estimates depend on the assumption that the percentage reductions in utilization would apply equally to all dialysis patients. However, this has not been substantiated.
Estimates also assumed a predictable, modest decline in ESA and IV iron utilization in the general US ESRD population continued in the year after the study (2012). Actual secular trends may modify these estimates. In addition to the savings we report here relating decreased ESA and IV iron usage, we also found that the use of FC was associated with a reduction in hospitalization rates and subsequent hospitalization costs, predicting a saving of $3002/patient/year when using FC [24]. The cost of binders is not accounted for in this analysis. It would be expected that FC would cost more than generic calcium acetate and sevelamer, and that this may offset some of the impact associated with the aforementioned savings predicted from decreased ESA and IV iron usage as well as the predicted savings associated with fewer hospitalizations. It was not the authors’ intent to model each and every one of these factors in this analysis, although it is planned to be done. This will require a more sophisticated pharmacoeconomic analysis considering all of these factors and should include a number of sensitivity analyses, especially since drug pricing and what patients actually pay are two different things altogether. Fortunately, the primary trial data reports the actual binder dosing requirements to achieve equivalent levels of serum phosphorus [16].
The primary trial was open label. The iron in FC causes the stools to become dark, and thus there was no way around this but to be open-label. Since both treatment groups in the primary trial had similar achieved phosphorus levels and essentially similar pill usage to achieve those levels (by pill counts), we do not feel that the open-label nature of the study impacted the reliability of the results [16]. The trial was based on 52 weeks of therapy. While there is no reason to believe that the efficacy of FC as a phosphorus binder would lessen beyond that time frame, longer term tolerability is yet to be determined.
Ferric citrate provides effective control of serum phosphorus in ESRD patients while increasing measures of iron [16, 17]. The accompanying reductions in the utilization of ESAs and IV iron in our study population suggest that FC may also prove to be a valuable tool for anemia management with the potential of providing cost savings to the health care system. Providing care to the ESRD patient costs $87,945 patient/year [18]. Patients with ESRD represent 1.4 % of Medicare patients but utilize 7.2 % of Medicare spending [18]. Thus, any maneuver that could decrease costs would be welcomed in our current economic environment.
References
Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: what is normal, when to start, and how to treat? Clin J Am Soc Nephrol. 2011;6(2):440–6.
Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant. 2011;26(2):402–7.
Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71(5):438–41.
Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24(5):1506–23.
Sinsakul M, Sika M, Koury M, Shapiro W, Greene T, Dwyer J, et al. The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract. 2012;121(1–2):c25–9.
Mohammed IA, Hutchison AJ. Phosphate binding therapy in dialysis patients: focus on lanthanum carbonate. Ther Clin Risk Manag. 2008;4(5):887–93.
Mutell R, Rubin J, Bond T, Mayne T. Reduced use of erythropoiesis-stimulating agents and intravenous iron with Ferric Citrate: a managed care cost-offset model. Int J Nephrol Renovasc Dis. 2013;6:79–87.
Umanath K, Sika M, Niecestro R, Connelly C, Schulman G, Koury MJ, et al. Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. Hemodial Int. 2013;17(1):67–74.
Dwyer JP, Sika M, Schulman G, Chang IJ, Anger M, Smith M, et al. Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis. 2013;61(5):759–66.
Arbor Research Collaborative for Health. 2012 Annual report of the Dialysis Outcomes and Practice Patterns Study: hemodialysis data 1997–2011. 2012 http://www.dopps.org/annualreport/ Accessed 23 May 2014.
Auryxia [Package Insert]. New York: Keryx Biopharmaceuticals Inc; 2014.
USRDS 2012 Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. 2012 http://www.usrds.org/atlas12.aspx Accessed 20 May 2014.
Fuller DS, Pisoni RL, Bieber B, Gillespie B, Robinson B. The DOPPS practice monitor for US Dialysis Care: update on trends in anemia management 2 years into the bundle. AJKD. 2013;61:342–346.
Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, et al. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18(3):975–84.
Kapoian T, O’Mara NB, Singh AK, Moran J, Rizkala AR, Geronemus R, et al. Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol. 2008;19(2):372–9.
Lewis J, Sika M, Koury M, Chuang P, Schulman G, Smith MT, et al. Ferric citrate controls phosphorus and delivers iron in dialysis patients. J Am Soc Nephrol. 2014;26:493–503.
Umanath K, Jalal D, Greco B, Umeukeje E, Reisin E, Manley J, et al. Ferric citrate reduces IV iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015 (in press).
USRDS 2013 Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. 2013 http://www.usrds.org/atlas.aspx. Accessed 20 May 2014.
Breslow N, Clayton D. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88(421):9–25.
ASP Pricing file. Q4 2013. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2013ASPFiles.html. Accessed Nov 2013.
ASP Pricing file. Q4 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2012ASPFiles.html. Accessed May 2014.
DaVita. Annual report. 2006 http://media.corporate-ir.net/media_files/irol/76/76556/reports/2006_AR.pdf Accessed 16 April 2014.
Fresenius Medical Care AG & Co. KGaA. Annual report pursuant to section 13 or 15(d) of the Securities Exchange Act. Filed 25 Feb 2014. http://www.fmcag.com/files/20F_2013.pdf. Accessed 16 April 2014.
Rodby R, Umanath K, Niecestro R, Jackson JH, Sika M, Lewis JB, et al. Phosphorus binding with ferric citrate is associated with fewer hospitalizations and reduced hospitalization costs. Expert Rev Pharmacoecon Outcomes Res. 2015;15:545–50.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Support and financial disclosure declaration
Roger Rodby, Kausik Umanath, Mohammed Sika, Jamie Dwyer, and Julia Lewis declare they have received research grants from, acted as consultants for, and received travel support from, Keryx Biopharmaceuticals, Inc. Robert Niecestro served as a compensated independent consultant for Keryx Biopharmaceuticals, Inc. T. Christopher Bond is a salaried employee of Covance Market Access, Inc. There are no potential conflicts of interest.
Keryx sponsored the underlying phase III clinical trial. Keryx provided Covance Market Access, Inc. financial support to conduct the data analysis for this present study. Some analyses presented here were previously presented in abstract and poster form at the 2014 NKF Spring Clinical Meeting in Las Vegas, NV, USA.
Design and conduct of clinical trial and data integrity: Roger A. Rodby, Kausik Umanath, Mohammed Sika, Jamie P. Dwyer, and Julia Lewis for the Collaborative Study Group, and Robert Niecestro, independent consultant; statistical analysis and manuscript composition: T. Christopher Bond, Covance Market Access, Inc. The Collaborative Study Group members above had a role in the composition and editing of the manuscript and the decision to submit the report for publication.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rodby, R.A., Umanath, K., Niecestro, R. et al. Ferric Citrate, an Iron-Based Phosphate Binder, Reduces Health Care Costs in Patients on Dialysis Based on Randomized Clinical Trial Data. Drugs R D 15, 271–279 (2015). https://doi.org/10.1007/s40268-015-0103-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-015-0103-y