Abstract
Aim
To find the incidence rate of adverse drug reactions (ADRs) and investigate its various aspects in patients admitted to surgery wards of a rural tertiary-care hospital in India.
Methodology
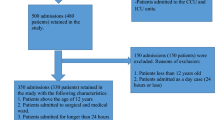
A prospective observational study, involving 800 patients over a period of 1.5 years, was carried out to find the incidence rate of ADRs, and various aspects of such events (e.g. causality, severity, preventability, causative drugs, organs/systems involved, and management strategy with outcome). A structured and pre-tested form was used to compile the data.
Results
An ADR was reported in 3.9 % of patients. Neither the age nor gender of the patients influenced incidence rate. Type A (augmented) reactions accounted for 83.9 % of ADRs. Causality assessment, using the WHO-UMC method, revealed that 58.1 and 41.9 % of ADRs fell into the ‘probable’ and ‘possible’ categories, respectively, whereas the corresponding proportions were 71.0 and 29.0 % using the Naranjo ADR probability scale. As the number of drugs per patient increased, the incidence of ADRs also increased. The majority (77.4 %) of ADRs were associated with antimicrobial drugs, followed by analgesics, with 71 % of ADRs involving the gastrointestinal system. No ADRs were fatal. Suspected drugs were discontinued in 64.5 % of patients and 96.8 % patients had fully recovered at the time of discharge.
Conclusion
Identification and monitoring of ADRs among various patient groups, including those admitted to general surgical wards of a hospital, along with meticulous reporting thereof, can help provide better and more rational patient care. Few studies that monitored ADRs in surgical patients are available. The incidence rate of ADRs amongst surgical patients in this Indian hospital appears to be much lower than commonly reported (3.9 vs. 10–25 %).
Similar content being viewed by others
References
Nebeker JR. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2005;140(10):795–801.
Tripathi KD. Essentials of medical pharmacology. 6th ed. New Delhi: Jaypee Publications.
ASHP guidelines on adverse drug reaction monitoring and reporting. American Society of Hospital Pharmacy. Am J Health Syst Pharm. 1995;52(4) 417–9.
Bates DW, Leape LL, Cullen DJ, et al. Effects of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–6.
Rodrigues GS, Khan SA. Pharmacovigilance among surgeons and in surgical wards: overlooked or axiomatic? Indian J Surg. 2011;73(1):4–8.
Farrokhi S, Nahvi H, Pourpak Z, et al. Adverse drug reactions in a department of pediatric surgery. J Trop Pediatr. 2006;52(1):72–3.
Davies EC, Green CF, Taylor S, et al. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009;4(2):e4439.
Boeker EB, de Boer M, Kiewiet JJ, et al. Occurrence and preventability of adverse drug events in surgical patients: a systematic review of literature. BMC Health Serv Res. 2013;13:364.
World Health Organization. The importance of pharmacovigilance: safety monitoring of medicinal products. Geneva: Office of Publications, World Health Organization; 2002.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–32.
Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6):538.
Central Drugs Standard Control Organization, Directorate General of Health Services, Ministry of Health & Family Welfare Government of India in collaboration with Indian Pharmacopoeia Commission. Pharmacovigilance programme of India (PvPI) for assuring drug safety. Ghaziabad: Central Drugs Standard Control Organization; 2011.
Gor AP, Desai SV. Adverse drug reactions (ADR) in the inpatients of medicine department of a rural tertiary care teaching hospital and influence of pharmacovigilance in reporting ADR. Indian J Pharmacol. 2008;40(1):37–40.
Sriram S, Ghasemi A, Ramasamy R, et al. Prevalence of adverse drug reactions at a private tertiary care hospital in south India. J Res Med Sci. 2011;16(1):16–25.
Rao PGM, Archana B, Jose J. Implementation and results of an adverse drug reaction reporting programme at an Indian teaching hospital. Indian J Pharmacol. 2006;38(4):293–4.
Arulmani R, Rajendran SD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol. 2008;65(2):210–6.
Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients-excess length of stay, extra costs and attributable mortality. JAMA. 1997;277(4):301–6.
Laurence DR, Bennett PN, Brown MJ. Clinical pharmacology. 8th ed. New York: Churchill Livingstone; 1997.
Sharma M, Gupta SK, Gupta VB. A comparative study of causality assessment scale used in the analysis of spontaneously reported events. J Pharmacovigil Drug Saf. 2009;6(1):5–9.
Hoigné R, Lawson DH, Weber E. Risk factor for adverse drug reactions: epidemiological approaches. Eur J Clin Pharmacol. 1990;39(4):321–5.
Holland EG, Degruy FV. Drug-induced disorders. Am Fam Physician. 1997;56(7):1791–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct the study or prepare this report.
Conflict of interest
Nirav N. Patel and Sagun V. Desai declare that they have no conflicts of interest relevant to the content of this manuscript.
Ethical approval
Ethical approval was obtained from the Institutional Ethics Committee before the commencement of the study. Written informed consent obtained from all the patients.
Rights and permissions
About this article
Cite this article
Patel, N., Desai, S. Profile of adverse drug reactions in patients admitted to general surgical wards of a rural tertiary-care hospital in India. Drugs Ther Perspect 31, 402–406 (2015). https://doi.org/10.1007/s40267-015-0231-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-015-0231-z