Abstract
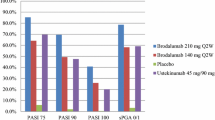
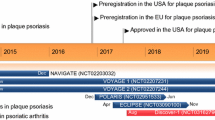
Brodalumab (Lumicef®) is a human monoclonal immunoglobulin G antibody that is being developed by Kyowa Hakko Kirin in Japan, where it has been approved for the treatment of psoriasis vulgaris, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. Brodalumab binds with high affinity to interleukin (IL)-17 receptor A, thereby inhibiting several pro-inflammatory cytokines from the IL-17 family. Regulatory applications for brodalumab in plaque psoriasis are also under review in the USA, EU and Canada. This article summarizes the milestones in the development of brodalumab leading to this first approval for the treatment of psoriasis.
Similar content being viewed by others
References
Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelb). 2016;6(1):1–12.
Kivelevitch DN, Menter A. Use of brodalumab for the treatment of psoriasis and psoriatic arthritis. Immunotherapy. 2015;7(4):323–33.
Kyowa Hakko Kirin. Lumicef® approved in Japan [media release]. 4 July 2016. http://www.kyowa-kirin.com.
Kyowa Hakko Kirin Co. Ltd. Lumicef® subcutaneous injection 210 mg syringe: brodalumab (gene recombination) formulation: Japanese prescribing information. 2016. http://www.kksmile.com. Accessed 5 Aug 2016.
Valeant Pharmaceuticals International. Valeant announces FDA acceptance of BLA submission for brodalumab in moderate-to-severe plaque psoriasis [media release]. 25 Jan 2016. http://www.valeant.com.
Farahnik B, Beroukhim K, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther (Heidelb). 2016;6(2):111–24.
FirstWord Pharma. FDA advisory panel backs Valeant’s psoriasis drug brodalumab, with suicide risk-mitigation plan [media release]. 19 July 2016. http://www.firstwordpharma.com.
Kyowa Hakko Kirin Co Ltd. Kyowa Hakko Kirin announces results of the phase 3 studies of KHK4827 in subjects with psoriasis in Japan [media release]. 23 July 2015. http://www.kyowa-kirin.com.
Amgen, AstraZeneca. Amgen and AstraZeneca announce collaboration to jointly develop and commercialize clinical-stage inflammation portfolio [media release]. 2 Apr 2012. http://www.astrazeneca.com.
Amgen. Amgen to terminate participation in co-development and commercialization of brodalumab [media release]. 22 May 2015. http://www.amgen.com.
AstraZeneca. AstraZeneca PLC H1 2015 results [media release]. 30 July 2015. http://www.astrazeneca.com.
Valeant Pharmaceuticals International. Valeant and AstraZeneca to partner on brodalumab [media release]. 1 Sep 2015. http://www.valeant.com.
AstraZeneca. AstraZeneca enters licensing agreements with LEO Pharma in skin diseases [media release]. 1 July 2016. http://www.astrazeneca.com.
Valeant Pharmaceuticals International. Valeant Pharmaceuticals announces new licensing arrangement for brodalumab in Europe [media release]. 1 July 2016. http://www.valeant.com.
Amgen. Form 10-K [media release]. 19 Feb 2016. http://investors.amgen.com.
Papp KA, Reid C, Foley P, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132(10):2466–9.
Salinger DH, Endres CJ, Martin DA, et al. A semi-mechanistic model to characterize the pharmacokinetics and pharmacodynamics of brodalumab in healthy volunteers and subjects with psoriasis in a first-in-human single ascending dose study. Clin Pharmacol Drug Dev. 2014;3(4):276–83.
Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828–36.
Endres CJ, Salinger DH, Kock K, et al. Population pharmacokinetics of brodalumab in healthy adults and adults with psoriasis from single and multiple dose studies. J Clin Pharmacol. 2014;54(11):1230–8.
Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44–52.
Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016. doi:10.1111/jdv.13785.
Yamasaki K, Nakagawa H. Clinical efficacy and safety of brodalumab (KHK4827), antiinterleukin-17-receptor a monoclonal antibody, in Japanese patients with pustular psoriasis (generalized) and psoriatic erythroderma: a phase 3, open-label, long-term study (4827-004 study) [abstract no. 365 plus poster]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB228.
Nakagawa H, Ootaki K. Long-term efficacy and safety of brodalumab in Japanese patients with plaque psoriasis (psoriasis vulgaris, psoriatic arthritis), pustular psoriasis (generalized) and psoriatic erythroderma: an open-label extension study [abstract no. P142]. J Eur Acad Dermatol Venereol. 2016;30(S6):72.
Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016. doi:10.1111/bjd.14493.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28.
Strober B, Gordon K, Augustin M, et al. Improvements in patient-reported outcomes (PROs) among moderate to severe plaque psoriasis patients treated with brodalumab: results from AMAGINE-1 [abstract no. 3169]. J Am Acad Dermatol. 2016;74(5 Suppl 1):AB256.
Paul C, Barker J, Klekotka P, et al. Improvements in health-related quality of life measured by SF-36 and EQ-5D in patients with moderate to severe plaque psoriasis treated with brodalumab [abstract no. 3172]. J Am Acad Dermatol. 2016;74(5 Suppl 1):AB256.
Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–9.
Papp K, Leonardi C, Menter A, et al. Maintenance of clinical response with long-term brodalumab (AMG 827) therapy for psoriasis: week 144 results from an open-label extension study [abstract no. 956]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB240.
Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–306.
Mease PJ, Genovese MC, Mutebi A, et al. Improvement in psoriasis signs and symptoms assessed by the psoriasis symptom inventory with brodalumab treatment in patients with psoriatic arthritis. J Rheumatol. 2016;43(2):343–9.
Genovese MC, Mease PJ, Greenwald MW, et al. Efficacy and safety of brodalumab over one year in patients with psoriatic arthritis with and without prior exposure to a biologic [abstract no. AB0752]. Ann Rheum Dis. 2014;73(Suppl 2):1052–3.
Mease P, Genovese MC, Greenwald MW, et al. Two-year clinical response to brodalumab, an anti-IL-17 receptor antibody, in patients with psoriatic arthritis [abstract no. OP0175]. Ann Rheum Dis. 2015;74(Supplement 2):136–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Sarah Greig is a salaried employee of Adis, Springer SBM.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
An erratum to this article can be found at http://dx.doi.org/10.1007/s40265-016-0647-3.
Rights and permissions
About this article
Cite this article
Greig, S.L. Brodalumab: First Global Approval. Drugs 76, 1403–1412 (2016). https://doi.org/10.1007/s40265-016-0634-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0634-8