Abstract
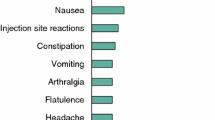
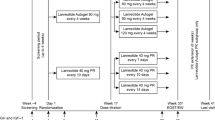
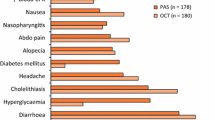
Lanreotide Autogel® (ATG) [Somatuline® Autogel®, Somatuline® Depot®] is a prolonged-release, supersaturated aqueous gel formulation of the somatostatin analogue lanreotide acetate that acts via somatostatin receptors to reduce both growth hormone and insulin-like growth factor-I levels. It is indicated for the treatment of patients with acromegaly who have had an inadequate response to or cannot be treated with surgery and/or radiotherapy. This article reviews the clinical efficacy and tolerability of lanreotide ATG in the treatment of acromegaly, as well as summarizing its pharmacological properties. Results of clinical trials and extension studies of up to 4 years duration showed that deep subcutaneous lanreotide ATG was a generally effective treatment in treatment-naive and treatment-experienced adults with acromegaly. Lanreotide ATG provided hormonal control and improved both health-related quality of life and acromegaly symptoms in most patients; it also reduced tumour volume to a clinically significant extent in studies of primary therapy. Moreover, lanreotide ATG was generally no less effective than intramuscular lanreotide long-acting microparticles and was as effective as intramuscular octreotide long-acting release in switching or crossover studies, including those with standard or extended dosing intervals. Lanreotide ATG is generally well tolerated; the most frequently reported adverse events were mild or moderate transient gastrointestinal symptoms. Lanreotide ATG also has the advantage of being available in a convenient pre-filled syringe and is given subcutaneously rather than intramuscularly. Thus, lanreotide ATG continues to be a valuable option in the treatment of acromegaly, with potential advantages being ease of administration and longer dosing intervals in patients who have an adequate response to initial therapy.


Similar content being viewed by others
References
Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355(24):2558–73.
Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract. 2011;17(Suppl 4):1–44.
Holdaway IM, Rajasoorya C. Epidemiology of acromegaly. Pituitary. 1999;2(1):29–41.
Melmed S, Colao A, Barkan A, et al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94(5):1509–17.
Feelders RA, Hofland LJ, Van Aken MO, et al. Medical therapy of acromegaly: efficacy and safety of somatostatin analogues. Drugs. 2009;69(16):2207–26.
Giustina A, Chanson P, Kleinberg D, et al. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10(4):243–8.
Ipsen Ltd. Somatuline® Autogel®, solution for injection in a pre-filled syringe: summary of product characteristics. 2011. http://www.medicines.org.uk/emc/medicine/25104/SPC/Somatuline+Autogel+60mg%2c+Somatuline+Autogel+90mg%2c+Somatuline+Autogel+120mg+New+device/. Accessed 19 June 2014.
Ipsen. Somatuline® Depot (lanreotide): US prescribing information. 2013. http://somatulinedepot.com. Accessed 19 June 2014.
Ministère des Affaires Sociales et de la Santé. SOMATULINE L.P. 120 mg solution for injection in a pre-filled syringe: summary of product characteristics. 2012. http://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=63787825&typedoc=R. Accessed 19 June 2014.
Croxtall JD, Scott LJ. Lanreotide Autogel: a review of its use in the management of acromegaly. Drugs. 2008;68(5):711–23.
Ciccarelli A, Daly A, Beckers A. Lanreotide Autogel for acromegaly: a new addition to the treatment armamentarium. Treat Endocrinol. 2004;3(2):77–81.
Patel YC, Sirikant CB. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1–5). Endocrinology. 1994;135(6):2814–7.
Krejs GJ. Physiological role of somatostatin in the digestive tract: gastric acid secretion, intestinal absorption, and motility. Scand J Gastroenterol Suppl. 1986;119:47–53.
Turner HE, Lindsell DRM, Vadivale A, et al. Differing effects on gall-bladder motility of lanreotide SR and octreotide LAR for treatment of acromegaly. Eur J Endocrinol. 1999;141(6):590–4.
Mazziotti G, Floriani I, Bonadonna S, et al. Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J Clin Endocrinol Metab. 2009;94(5):1500–8.
Castinetti F, Saveanu A, Morange I, et al. Lanreotide for the treatment of acromegaly. Adv Ther. 2009;26(6):600–12.
Gasco V, Beccuti G, Marotta F, et al. Effects of chronic slow release-lanreotide treatment on insulin-like growth factor system and metabolic parameters in acromegalic patients. J Endocrinol Invest. 2012;35(4):372–7.
Steffin B, Gutt B, Bidlingmaier M, et al. Effects of the long-acting somatostatin analogue lanreotide Autogel on glucose tolerance and insulin resistance in acromegaly. Eur J Endocrinol. 2006;155(1):73–8.
Couture E, Bongard V, Maiza J-C, et al. Glucose status in patients with acromegaly receiving primary treatment with the somatostatin analog lanreotide. Pituitary. 2012;15(4):518–25.
Ning S, Knox SJ, Harsh GR, et al. Lanreotide promotes apoptosis and is not radioprotective in GH3 cells. Endocr Relat Cancer. 2009;16(3):1045–55.
Zatelli MC, Ambrosio MR, Bondanelli M, et al. Control of pituitary adenoma cell proliferation by somatostatin analogs, dopamine agonists and novel chimeric compounds. Eur J Endocrinol. 2007;156(Suppl. 1):S29–35.
Florio T, Thellung S, Arena S, et al. Somatostatin and its analog lanreotide inhibit the proliferation of dispersed human non-functioning pituitary adenoma cells in vitro. Eur J Endocrinol. 1999;141(4):396–408.
Mazziotti G, Giustina A. Effects of lanreotide SR and Autogel on tumor mass in patients with acromegaly: a systematic review. Pituitary. 2010;13(1):60–7.
Caron PJ, Bevan JS, Petersenn S, et al. Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J Clin Endocrinol Metab. 2013;99(4):1282–90.
Miller GM, Alexander JM, Bikkal HA, et al. Somatostatin receptor subtype gene expression in pituitary adenomas. J Clin Endocrinol Metab. 1995;80(4):1386–92.
Sideris L, Dube P, Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist. 2012;17(6):747–55.
Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol. 2013;40(1):56–68.
Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–33.
Sacca L, Cittadini A, Fazio S. Growth hormone and the heart. Endocr Rev. 1994;15(5):555–73.
Brevetti G, Marzullo P, Silvestro A, et al. Early vascular alterations in acromegaly. J Clin Endocrinol Metab. 2002;87(7):3174–9.
Manelli F, Desenzani P, Boni E, et al. Cardiovascular effects of a single slow release lanreotide injection in patients with acromegaly and left ventricular hypertrophy. Pituitary. 1999;2(3):205–10.
Hradec J, Kral J, Janota T, et al. Regression of acromegalic left ventricular hypertrophy after lanreotide (a slow-release somatostatin analog). Am J Cardiol. 1999;83(10):1506–9.
Baldelli R, Ferretti E, Jaffrain-Rea ML, et al. Cardiac effects of slow-release lanreotide, a slow-release somatostatin analog, in acromegalic patients. J Clin Endocrinol Metab. 1999;84(2):527–32.
Colao A, Marek J, Goth MI, et al. No greater incidence or worsening of cardiac valve regurgitation with somatostatin analog treatment of acromegaly. J Clin Endocrinol Metab. 2008;93(6):2243–8.
Colao A, Auriemma RS, Galdiero M, et al. Effects of initial therapy for five years with somatostatin analogs for acromegaly on growth hormone and insulin-like growth factor-I levels, tumor shrinkage, and cardiovascular disease: a prospective study. J Clin Endocrinol Metab. 2009;94(10):3746–56.
Colao A, Marzullo P, Lombardi G. Effect of a six-month treatment with lanreotide on cardiovascular risk factors and arterial intima-media thickness in patients with acromegaly. Eur J Endocrinol. 2002;146(3):303–9.
Lombardi G, Colao A, Marzullo P, et al. Improvement of left ventricular hypertrophy and arrhythmias after lanreotide-induced GH and IGF-I decrease in acromegaly: a prospective multi-center study. J Endocrinol Invest. 2002;25(11):971–6.
Annamalai AK, Webb A, Kandasamy N, et al. A comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapy. J Clin Endocrinol Metab. 2013;98(3):1040–50.
Cendros JM, Peraire C, Troconiz IF, et al. Pharmacokinetics and population pharmacodynamic analysis of lanreotide Autogel. Metabolism. 2005;54(10):1276–81.
Bronstein M, Musolino N, Jallad R, et al. Pharmacokinetic profile of lanreotide Autogel in patients with acromegaly after four deep subcutaneous injections of 60, 90 or 120 mg every 28 days. Clin Endocrinol (Oxf). 2005;63(5):514–9.
Antonijoan RM, Barbanoj MJ, Cordero JA, et al. Pharmacokinetics of a new Autogel formulation of the somatostatin analogue lanreotide after a single subcutaneous dose in healthy volunteers. J Pharm Pharmacol. 2004;56(4):471–6.
Gomez-Panzani E, Chang S, Ramis J. Sustained biochemical control in patients with acromegaly treated with lanreotide depot 120 mg administered every 4 weeks, or an extended dosing interval of 6 or 8 weeks: a pharmacokinetic approach. Res Rep Endocr Disord. 2012;2:79–84.
Troconiz IF, Cendros J-M, Peraire C, et al. Population pharmacokinetic analysis of lanreotide Autogel in healthy subjects: evidence for injection interval of up to 2 months. Clin Pharmacokinet. 2009;48(1):51–62.
Shimatsu A, Teramoto A, Hizuka N, et al. Efficacy, safety, and pharmacokinetics of sustained-release lanreotide (lanreotide Autogel) in Japanese patients with acromegaly or pituitary gigantism. Endocr J. 2013;60(5):651–63.
Giustina A, Chanson P, Bronstein MD, et al. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95(7):3141–8.
Lombardi G, Minuto F, Tamburrano G, et al. Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in somatostatin analogue-naive patients with acromegaly. J Endocrinol Invest. 2009;32(3):202–9.
Caron P, Bex M, Cullen DR, et al. One-year follow-up of patients with acromegaly treated with fixed or titrated doses of lanreotide Autogel. Clin Endocrinol (Oxf). 2004;60(6):734–40.
Caron P, Beckers A, Cullen DR, et al. Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegaly. J Clin Endocrinol Metab. 2002;87(1):99–104.
Lucas T, Astorga R, Spanish-Portuguese Multicentre Autogel Study Group on Acromegaly. Efficacy of lanreotide Autogel administered every 4–8 weeks in patients with acromegaly previously responsive to lanreotide microparticles 30 mg: a phase III trial. Clin Endocrinol (Oxf). 2006;65(3):320–6.
Chanson P, Borson-Chazot F, Kuhn J-M, et al. Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly. Clin Endocrinol (Oxf). 2008;69(2):299–305.
Melmed S, Cook D, Schopohl J, et al. Rapid and sustained reduction of serum growth hormone and insulin-like growth factor-1 in patients with acromegaly receiving lanreotide Autogel therapy: a randomized, placebo-controlled, multicenter study with a 52 week open extension. Pituitary. 2010;13(1):18–28.
Ronchi CL, Boschetti M, Degli Uberti EC, et al. Efficacy of a slow-release formulation of lanreotide (Autogel) 120 mg in patients with acromegaly previously treated with octreotide long acting release (LAR): an open, multicentre longitudinal study. Clin Endocrinol (Oxf). 2007;67(4):512–9.
Schopohl J, Strasburger CJ, Caird D, et al. Efficacy and acceptability of lanreotide Autogel 120 mg at different dose intervals in patients with acromegaly previously treated with octreotide LAR. Exp Clin Endocrinol Diabetes. 2011;119(3):156–62.
Alexopoulou O, Abrams P, Verhelst J, et al. Efficacy and tolerability of lanreotide Autogel therapy in acromegalic patients previously treated with octreotide LAR. Eur J Endocrinol. 2004;151(3):317–24.
Caron P, Cogne M, Raingeard I, et al. Effectiveness and tolerability of 3-year lanreotide Autogel treatment in patients with acromegaly. Clin Endocrinol (Oxf). 2006;64(2):209–14.
Gutt B, Bidlingmaier M, Kretschmar K, et al. Four-year follow-up of acromegalic patients treated with the new long-acting formulation of lanreotide (lanreotide Autogel). Exp Clin Endocrinol Diabetes. 2005;113(3):139–44.
Andries M, Glintborg D, Kvistborg A, et al. A 12-month randomized crossover study on the effects of lanreotide Autogel and octreotide long-acting repeatable on GH and IGF-l in patients with acromegaly. Clin Endocrinol (Oxf). 2008;68(3):473–80.
van der Lelij A-J, Pronin V, Balcere I, et al. Efficacy and safety of lanreotide Autogel 120 mg at extended dosing intervals in acromegalic patients biochemically controlled with octreotide LAR 10 or 20 mg: the LEAD study [abstract no. P912]. 16th European Congress of Endocrinology, Wrocław; 3–7 May 2014.
van der Lely A-J, Bernabeu I, Cap J, et al. Coadministration of lanreotide Autogel and pegvisomant normalizes IGF1 levels and is well tolerated in patients with acromegaly partially controlled by somatostatin analogs alone. Eur J Endocrinol. 2011;164(3):325–33.
Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159(2):89–95.
Dekkers OM, Biermasz NR, Pereira AM, et al. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2008;93(1):61–7.
Mao Z-g, Zhu Y-h, Tang H-l, et al. Preoperative lanreotide treatment in acromegalic patients with macroadenomas increases short-term postoperative cure rates: a prospective, randomised trial. Eur J Endocrinol. 2010;162(4):661–6.
Lucas T, Astorga R, Catala M, et al. Preoperative lanreotide treatment for GH-secreting pituitary adenomas: effect on tumour volume and predictive factors of significant tumour shrinkage. Clin Endocrinol (Oxf). 2003;58(4):471–81.
Abrams P, Alexopoulou O, Abs R, et al. Optimalization and cost management of lanreotide-Autogel therapy in acromegaly. Eur J Endocrinol. 2007;157(5):571–7.
Marzullo P, Ferone D, Di Somma C, et al. Efficacy of combined treatment with lanreotide and cabergoline in selected therapy-resistant acromegalic patients. Pituitary. 1999;1(2):115–20.
Boyd AE, DeFord LL, Mares JE, et al. Improving the success rate of gluteal intramuscular injections. Pancreas. 2013;42(5):878–82.
Bevan JS, Newell-Price J, Wass JAH, et al. Home administration of lanreotide Autogel by patients with acromegaly, or their partners, is safe and effective. Clin Endocrinol (Oxf). 2008;68(3):343–9.
Salvatori R, Nachtigall LB, Cook DM, et al. Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naive patients with acromegaly. Pituitary. 2010;13(2):115–22.
Salvatori R, Woodmansee WW, Molitch M, et al. Lanreotide extended-release aqueous-gel formulation, injected by patient, partner or healthcare provider in patients with acromegaly in the United States: 1-year data from the SODA registry. Pituitary. 2013;17(1):13–21.
Adelman DT, Burgess A, Davies PR. Evaluation of long-acting somatostatin analog injection devices by nurses: a quantitative study. Med Devices (Auckl). 2012;5:103–9.
Orlewska E, Kos-Kudla B, Sowinski J, et al. Assessment of real-world usage of lanreotide Autogel 120 in Polish acromegalic patients - results from the prospective 12-month phase of Lanro-Study. Contemp Oncol (Pozn). 2013;17(5):460–5.
Marty R, Roze S, Kurth H. Decision-tree model for health economic comparison of two long-acting somatostatin receptor ligand devices in France, Germany, and the UK. Med Devices (Auckl). 2012;5:39–44.
Valentim J, Passos V, Mataveli F, et al. Cost-effectiveness analysis of somatostatin analogues in the treatment of acromegaly in Brazil. Arq Bras Endocrinol Metabol. 2008;52(9):1452–60.
Gadelha M, Bronstein M, Brue T, et al. Pasireotide LAR demonstrates superior efficacy versus octreotide LAR and lanreotide ATG in patients with inadequately controlled acromegaly: results from a phase III, multicentre, randomized study (PAOLA) [abstract no. P907]. 16th European Congress of Endocrinology, Wrocław; 3–7 May 2014.
Chiasma I. Efficacy and safety of oral Octreolin™ in patients with acromegaly who are currently receiving parenteral somatostatin analogs [ClinicalTrials.gov identifier NCT01412424]. US National Institutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT01412424. Accessed 19 June 2014.
Aspireo Pharmaceuticals. Somatoprim. 2014. http://www.aspireopharma.com/somatoprim/. Accessed 19 June 2014.
Garrido MJ, Cendros J-M, Ramis J, et al. Pharmacodynamic modeling of the effects of lanreotide Autogel on growth hormone and insulin-like growth factor 1. J Clin Pharmacol. 2012;52(4):487–98.
Colao A, Marzullo P, Vallone G, et al. Ultrasonographic evidence of joint thickening reversibility in acromegalic patients treated with lanreotide for 12 months. Clin Endocrinol (Oxf). 1999;51(5):611–8.
Cappelli C, Gandossi E, Agosti B, et al. Long-term treatment of acromegaly with lanreotide: evidence of increased serum parathormone concentration. Endocr J. 2004;51(6):517–20.
Bevan JS. Clinical review: the antitumoral effects of somatostatin analog therapy in acromegaly. J Clin Endocrinol Metab. 2005;90(3):1856–63.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. Celeste Burness, Sohita Dhillon and Susan Keam are salaried employees of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: M. Bronstein, University of Sao Paulo Medical School, Sao Paulo, Brazil; P. Chanson, Publique-Hôpitaux de Paris, Hospital Bicetre and University Paris XI, Le Kremlin-Bicetre, France; A. L. Kennedy, Cleveland Clinic Foundation, Cleveland, Ohio, USA.
Rights and permissions
About this article
Cite this article
Burness, C.B., Dhillon, S. & Keam, S.J. Lanreotide Autogel®: A Review of its Use in the Treatment of Patients with Acromegaly. Drugs 74, 1673–1691 (2014). https://doi.org/10.1007/s40265-014-0283-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0283-8