Abstract
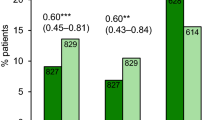
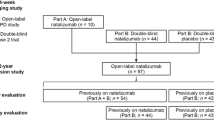
Natalizumab (Tysabri®) is a humanized monoclonal antibody against the α4 chain of integrins and was the first targeted therapy to be approved for the treatment of relapsing-remitting multiple sclerosis (RRMS). Natalizumab acts as a selective adhesion molecule antagonist, which binds very late antigen (VLA)-4 and inhibits the translocation of activated VLA-4-expressing leukocytes across the blood–brain barrier into the CNS. In a pivotal phase III clinical trial, natalizumab 300 mg intravenously every 4 weeks for 2 years in adults with RRMS significantly reduced the annualized relapse rate and the risk of sustained progression of disability compared with placebo, as well as significantly increasing the proportion of relapse-free patients at 1 and 2 years. Natalizumab also significantly reduced the number of T2-hyperintense, gadolinium-enhancing and T1-hypointense lesions on magnetic resonance imaging, and significantly reduced the volume of T2-hyperintense and T1-hypointense lesions compared with placebo. Natalizumab recipients generally experienced improved health-related quality of life at 1–2 years. Natalizumab was generally well tolerated in pivotal trials. The only adverse events that were more frequent with natalizumab monotherapy than with placebo were fatigue and allergic reactions. The main safety and tolerability issue with natalizumab is the incidence of progressive multifocal leukoencephalopathy (PML). As long as the risk of PML is managed effectively, natalizumab is a valuable therapeutic option for adults with highly active relapsing forms of multiple sclerosis.
Similar content being viewed by others
References
Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–8.
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17.
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17(2):210–8.
The Merck Manual for health care professionals. Multiple sclerosis (MS). 2007. http://www.merckmanuals.com/professional/neurologic_disorders/demyelinating_disorders/multiple_sclerosis_ms.html#v1045059. Accessed 12 Jul 2013.
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–31.
Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356(25):2603–13.
Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt. 7):1914–29.
Goodin DS, Frohman EM, Garmany GP Jr, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58(2):169–78.
Freedman MS, Blumhardt LD, Brochet B, et al. International consensus statement on the use of disease-modifying agents in multiple sclerosis. Mult Scler. 2002;8(1):19–23.
Menge T, Weber MS, Hemmer B, et al. Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs. 2008;68(17):2445–68.
European Medicines Agency. Tysabri (natalizumab): summary of product characteristics. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf. Accessed 12 Jul 2013.
Gensicke H, Leppert D, Yaldizli O, et al. Monoclonal antibodies and recombinant immunoglobulins for the treatment of multiple sclerosis. CNS Drugs. 2012;26(1):11–37.
European Medicines Agency. Tysabri (natalizumab): summary of opinion (post authorisation). 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000603/smops/Positive/human_smop_000525.jsp&mid=WC0b01ac058001d127. Accessed 12 Jul 2013.
Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135(4):268–75.
Oreja-Guevara C, Ramos-Cejudo J, Aroeira LS, et al. TH1/TH2 cytokine profile in relapsing-remitting multiple sclerosis patients treated with glatiramer acetate or natalizumab. BMC Neurol. 2012;12:95.
Biogen Idec, Elan Pharmaceuticals. Tysabri (natalizumab) injection: US prescribing information. 2012. http://www.tysabri.com/pdfs/I61061-13_PI.pdf. Accessed 12 Jul 2013.
Soon D, Altmann DR, Fernando KTM, et al. A study of subtle blood brain barrier disruption in a placebo-controlled trial of natalizumab in relapsing remitting multiple sclerosis. J Neurol. 2007;254(3):306–14.
Stüve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59(5):743–7.
Stüve O, Marra CM, Bar-Or A, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol. 2006;63(10):1383–7.
del Pilar Martin M, Cravens PD, Winger R, et al. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch Neurol. 2008;65(12):1596–603.
de Andrés C, Teijeiro R, Alonso B, et al. Long-term decrease in VLA-4 expression and functional impairment of dendritic cells during natalizumab therapy in patients with multiple sclerosis. PLoS ONE. 2012;7(4):e34103.
Millonig A, Hegen H, Di Pauli F, et al. Natalizumab treatment reduces endothelial activity in MS patients. J Neuroimmunol. 2010;227(1–2):190–4.
Khademi M, Stol D, Olsson T, et al. Induction of systemic TNFα in natalizumab-treated multiple sclerosis. Eur J Neurol. 2008;15(3):309–12.
Kivisäkk P, Healy BC, Viglietta V, et al. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology. 2009;72(22):1922–30.
Börnsen L, Christensen JR, Ratzer R, et al. Effect of natalizumab on circulating CD4+ T-cells in multiple sclerosis. PLoS ONE. 2012;7(11):e47578.
Benkert TF, Dietz L, Hartmann EM, et al. Natalizumab exerts direct signaling capacity and supports a pro-inflammatory phenotype in some patients with multiple sclerosis. PLoS ONE. 2012;7(12):e52208.
Krumbholz M, Meinl I, Kümpfel T, et al. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology. 2008;71(17):1350–4.
Planas R, Jelčić I, Schippling S, et al. Natalizumab treatment perturbs memory- and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur J Immunol. 2012;42(3):790–8.
Skarica M, Eckstein C, Whartenby KA, et al. Novel mechanisms of immune modulation of natalizumab in multiple sclerosis patients. J Neuroimmunol. 2011;235(1–2):70–6.
Bonig H, Wundes A, Chang K-H, et al. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111(7):3439–41.
Jing D, Oelschlaegel U, Ordemann R, et al. CD49d blockade by natalizumab in patients with multiple sclerosis affects steady-state hematopoiesis and mobilizes progenitors with a distinct phenotype and function. Bone Marrow Transplant. 2010;45(10):1489–96.
Saure C, Warnke C, Zohren F, et al. Natalizumab and impedance of the homing of CD34+ hematopoietic progenitors. Arch Neurol. 2011;68(11):1428–31.
Cree B, De Seze J, Fox R, et al. RESTORE study: effects of a 24-week natalizumab treatment interruption on immune parameters and multiple sclerosis magnetic resonance imaging disease activity [abstract]. Neurology. 2012;78(Suppl):1.
Gunnarsson M, Malmeström C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69(1):83–9.
Schneider H, Weber CE, Hellwig K, et al. Natalizumab treatment during pregnancy—effects on the neonatal immune system. Acta Neurol Scand. 2013;127(1):e1–4.
Sheremata WA, Vollmer TL, Stone LA, et al. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology. 1999;52(5):1072–4.
Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72(5):402–9.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–23.
Biogen Idec, Elan Pharmaceuticals. Natalizumab re-initiation of dosing (ClinicalTrials.gov identifier NCT00306592) US National Institutes of Health, ClinicalTrials.gov. 2009. http://clinicaltrials.gov/ct2/show/NCT00306592?term=natalizumab+AND+sclerosis&rank=18. Accessed 12 Jul 2013.
Biogen Idec, Elan Pharmaceuticals. Natalizumab re-initiation of dosing (STRATA) [ClinicalTrials.gov identifier NCT00297232] US National Institutes of Health, ClinicalTrials.gov. 2012. http://clinicaltrials.gov/ct2/show/NCT00297232?term=nct00297232&rank=1. Accessed 12 Jul 2013.
Biogen Idec. TOP: IMA-06-02 tysabri obervational program (ClinicalTrials.gov identifier NCT00493298) US National Institutes of Health, ClinicalTrials.gov. 2012. http://clinicaltrials.gov/ct2/show/NCT00493298?term=nct00493298&rank=1. Accessed 12 Jul 2013.
Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390–401.
Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8(3):254–60.
Balcer LJ, Galetta SL, Polman CH, et al. Low-contrast acuity measures visual improvement in phase 3 trial of natalizumab in relapsing MS. J Neurol Sci. 2012;318(1–2):119–24.
Balcer LJ, Galetta SL, Calabresi PA, et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68(16):1299–304.
Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62(4):335–46.
Kamat SA, Rajagopalan K, Stephenson JJ, et al. Impact of natalizumab on patient-reported outcomes in a clinical practice setting: a cross-sectional survey. Patient. 2009;2(2):105–12.
Olofsson S, Wickström A, Häger Glenngård A, et al. Effect of treatment with natalizumab on ability to work in people with multiple sclerosis: productivity gain based on direct measurement of work capacity before and after 1 year of treatment. BioDrugs. 2011;25(5):299–306.
Goodman A, O’Connor P, Polman C, et al. Updated safety and efficacy of natalizumab in the ongoing STRATA study [abstract]. Mult Scler. 2011;17(10 Suppl. 1):S444–5.
Polman C, Goodman A, Kappos L, et al. Efficacy and safety of natalizumab in the STRATA study [abstract no. P06.173]. 62nd Annual Meeting of the American Academy of Neurology; 10-17 Apr 2010; Toronto, ON.
Rudick R, Kappos L, Polman C, et al. Long-term outcomes in natalizumab-treated patients who were free of disease activity over the 2-year AFFIRM study [abstract]. Mult Scler. 2011; 17(10 Suppl. 1):S219–20.
Kappos L, Belachew S, Butzkueven H, et al. Long-term safety and efficacy and association between baseline treatment history and postbaseline relapses in multiple sclerosis patients treated with natalizumab in the Tysabri observational program (TOP) [abstract]. Neurology. 2012;78(Suppl):1.
Panzara MA, Bozic C, Sandrock AW. More on melanoma with transdifferentiation. N Engl J Med. 2008;359(1):99–100.
Castela E, Lebrun-Frenay C, Laffon M, et al. Evolution of nevi during treatment with natalizumab: a prospective follow-up of patients treated with natalizumab for multiple sclerosis. Arch Dermatol. 2011;147(1):72–6.
Bezabeh S, Flowers CM, Kortepeter C, et al. Clinically significant liver injury in patients treated with natalizumab. Aliment Pharmacol Ther. 2010;31:1028–35.
Jensen PEH, Koch-Henriksen N, Sellebjerg F, et al. Prediction of antibody persistency from antibody titres to natalizumab. Mult Scler. 2012;18(10):1493–9.
Holmén C, Piehl F, Hillert J, et al. A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler. 2011;17(6):708–19.
Oliver B, Fernández O, Orpez T, et al. Kinetics and incidence of anti-natalizumab antibodies in multiple sclerosis patients on treatment for 18 months. Mult Scler. 2011;17(3):368–71.
Piehl F, Holmén C, Hillert J, et al. Swedish natalizumab (Tysabri) multiple sclerosis surveillance study. Neurol Sci. 2011;31(Suppl. 3):S289–93.
Sørensen PS, Jensen PEH, Haghikia A, et al. Occurrence of antibodies against natalizumab in relapsing multiple sclerosis patients treated with natalizumab. Mult Scler. 2011;17(9):1074–8.
Lundkvist M, Engdahl E, Holmén C, et al. Characterization of anti-natalizumab antibodies in multiple sclerosis patients. Mult Scler. 2013;19(6):757–64.
Vennegoor A, Rispens T, Strijbis EM, et al. Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult Scler. 2013;19(5):593–600.
Calabresi PA, Giovannoni G, Confavreux C, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology. 2007;69(14):1391–403.
Becher B. Central nervous system immune surveillance: on natalizumab, dendritic cells, and dangerous immune privilege. Arch Neurol. 2008;65(12):1566–7.
Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–37.
Kappos L, Bates D, Edan G, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10(8):745–58.
Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–80.
Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353(4):362–8.
Biogen Idec, Elan Corporation plc. New Tysabri data reaffirm substantial efficacy in treatment of people with MS and demonstrate stability of anti-JCV antibody status [media release]. 2013. http://www.biogenidec.com/PRESS_RELEASE_DETAILS.aspx?ID=5981&ReqId=1797077.
Barts and The London School of Medicine and Dentistry. Mulitple sclerosis research: natalizumab-associated PML update: March 2013. 2013. http://multiple-sclerosis-research.blogspot.co.nz/2013/04/natalizumab-associated-pml-update-march.html. Accessed 12 Jul 2013.
Fernández Ó. Best practice in the use of natalizumab in multiple sclerosis. Ther Adv Neurol Disord. 2013;6(2):69–79.
Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JCV antibody index further defines PML risk in natalizumab-treated MS patients [abstract no. DX51 plus poster]. In: 27th Annual Meeting of the Consortium of Multiple Sclerosis Centers; 29 May–1 Jun 2013; Orlando, FL.
European Medicines Agency. Transatlantic workshop on drug-related progressive multifocal leukoencephalopathy (PML): workshop proceedings. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2011/09/WC500111562.pdf. Accessed 12 Jul 2013.
Foley J. Natalizumab related PML: an evolving risk stratification paradigm [abstract no. S30.002]. In: 65th Annual Meeting of the American Academy of Neurology; 16-23 March 2013; San Diego, CA.
Perkins MR, Ryschkewitsch C, Liebner JC, et al. Changes in JC virus-specific T cell responses during natalizumab treatment and in natalizumab-associated progressive multifocal leukoencephalopathy. PLoS Pathog. 2012;8(11):e1003014.
O’Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology. 2011;76(22):1858–65.
Tubridy N, Behan PO, Capildeo R, et al. The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology. 1999;53(3):466–72.
Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol. 2011;68(2):186–91.
Vellinga MM, Castelijns JA, Barkhof F, et al. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008; 70(13 Pt. 2):1150–1.
Borriello G, Prosperini L, Mancinelli C, et al. Pulse monthly steroids during an elective interruption of natalizumab: a post-marketing study. Eur J Neurol. 2012;19(5):783–7.
Berger JR, Centonze D, Comi G, et al. Considerations on discontinuing natalizumab for the treatment of multiple sclerosis. Ann Neurol. 2010;68(3):409–11.
Havla J, Tackenberg B, Hellwig K, et al. Early initiation of fingolimod treatment reduces the recurrence of disease activity after natalizumab discontinuation in multiple sclerosis [abstract no. P01.199 plus poster]. In: 65th Annual Meeting of the American Academy of Neurology; 16-23 Mar 2013; San Diego, CA.
LaGanke CC, Cullman AL. Natalizumab exit strategy experience with fingolimod [abstract no. P01.206]. In: 65th Annual Meeting of the American Academy of Neurology; 16–23 Mar 2013; San Diego, CA.
Cohen M, Maillart E, Papeix C, et al. ENIGM: a French observational study about switching from natalizumab to fingolimod in multiple sclerosis [abstract no. S41.002]. In: 65th Annual Meeting of the American Academy of Neurology; 16–23 Mar 2013; San Diego, CA.
Earnshaw SR, Graham J, Oleen-Burkey M, et al. Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Health Policy. 2009;7(2):91–108.
Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri®) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26(7):617–27.
Kobelt G, Berg J, Lindgren P, et al. Modeling the cost-effectiveness of a new treatment for MS (natalizumab) compared with current standard practice in Sweden. Mult Scler. 2008;14(5):679–90.
Hellwig K, Haghikia A, Gold R. Pregnancy and natalizumab: results of an observational study in 35 accidental pregnancies during natalizumab treatment. Mult Scler. 2011;17(8):958–63.
Lu E, Wang BW, Guimond C, et al. Safety of disease-modifying drugs for multiple sclerosis in pregnancy: current challenges and future considerations for effective pharmacovigilance. Expert Rev Neurother. 2013;13(3):251–60.
National Collaborating Centre for Chronic Conditions. Multiple sclerosis: national clinical guideline for diagnosis and management in primary and secondary care (NICE Clinical Guideline, No. 8). 2004. http://www.ncbi.nlm.nih.gov/books/NBK48919/pdf/TOC.pdf. Accessed 12 Jul 2013.
Association of British Neurologists. Revised (2009) Association of British Neurologists’ guidelines for prescribing in multiple sclerosis. 2009. http://www.theabn.org/abn/userfiles/file/abn_ms_guidelines_2009_final.pdf. Accessed 12 Jul 2013.
National Clinical Advisory Board of the National Multiple Sclerosis Society. Disease management concensus statement about use of current disease-modifying agents. 2008. http://www.nationalmssociety.org/ms-clinical-care-network/clinical-resources-and-tools/publications/expert-opinion-papers/index.aspx. Accessed 12 Jul 2013.
Rudick RA, Polman CH. Current approaches to the identification and management of breakthrough disease in patients with multiple sclerosis. Lancet Neurol. 2009;8(6):545–59.
Scott LJ. Fingolimod: a review of its use in the management of relapsing-remitting multiple sclerosis. CNS Drugs. 2011;25(8):673–98.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15.
European Medicines Agency. Gilenya (fingolimod): summary of product characteristics. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf. Accessed 12 Jul 2013.
European Medicines Agency. Gilenya (fingolimod): EPAR summary for the public. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002202/WC500104530.pdf. Accessed 12 Jul 2013.
Ali R, Nicholas RS, Muraro PA. Drugs in development for relapsing multiple sclerosis. Drugs. 2013;73(7):625–50.
Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97.
Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107.
European Medicines Agency. Tecfidera (dimethyl fumarate): summary of opinion (initial authorisation). 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002601/WC500140695.pdf. Accessed 12 Jul 2013.
European Medicines Agency. Aubagio (teriflunomide): summary of opinion (initial authorisation). 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002514/WC500140690.pdf. Accessed 12 Jul 2013.
Miller AE, O’Connor P, Wolinsky JS, et al. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult Scler. 2012;18(11):1625–32.
Comi G, Jeffery D, Kappos L, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med. 2012;366(11):1000–9.
Vollmer TL, Soelberg Sorensen P, Arnold DL, et al. A placebo-controlled and active comparator phase III trial (BRAVO) for relapsing-remitting multiple sclerosis [abstract no. 148]. In: 5th Joint triennial congress of the European and Americas Committees for Treatment and Research in Multiple Sclerosis; 19–22 Oct 2011; Amsterdam.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39.
Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9884):2167–75.
Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–87.
Tremlett H, Zhu F, Petkau J, et al. Natural, innate improvements in multiple sclerosis disability. Mult Scler. 2012;18(10):1412–21.
Sharac J, McCrone P, Sabes-Figuera R. Pharmacoeconomic considerations in the treatment of multiple sclerosis. Drugs. 2010;70(13):1677–91.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: A. Chaudhuri, Department of Neurology, Queen’s Hospital, Romford, London, UK; O. Fernández, Service of Neurology, Institute of Neurosciences, Hospital Regional Universitario Carlos Haya, Malaga, Spain; H. Lassmann, Center for Brain Research, Medical University of Vienna, Vienna, Austria; T. Menge, Department of Neurology, Heinrich-Heine University, Düsseldorf, Germany; F. Piehl, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; W.A. Sheremata, Department of Neurology, Multiple Sclerosis Center of Excellence, Miller School of Medicine, University of Miami, Miami, FL, USA.
Rights and permissions
About this article
Cite this article
McCormack, P.L. Natalizumab: A Review of Its Use in the Management of Relapsing-Remitting Multiple Sclerosis. Drugs 73, 1463–1481 (2013). https://doi.org/10.1007/s40265-013-0102-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0102-7