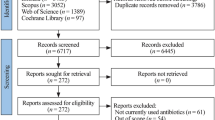
Abstract
Introduction
Reporting of harms in randomized control trials is often inconsistent and inadequate.
Objective
To assess the quality of harms reporting in randomized control trials evaluating the efficacy of antibiotics used to treat pediatric acute otitis media and to investigate whether connections to pharmaceutical companies or the publication of the CONSORT-Harms extension influenced the quality of harms reporting.
Study design and setting
We considered randomized control trials that evaluated the efficacy and safety of antibiotic treatment for uncomplicated acute otitis media in children aged 0–19. We evaluated the quality of harms reporting using a 19-item checklist addressing the recommendations endorsed in the CONSORT-Harms extension.
Results
160 studies met our inclusion criteria. Overall quality of reporting relating to harms was low; on average studies adhered to 55.2% of the checklist items on the quality of harms reporting. The reporting of methods relating the measurement of harms was particularly lacking; studies adhered to an average of only 33.2% of the checklist items. The overall quality of reporting did not change after the publication of the CONSORT-Harms extension. The overall quality of reporting did not differ significantly in reports with or without declared connections to pharmaceutical companies (mean quality score of 56.8% vs 52.0%, respectively).
Conclusions
Harms reporting in pediatric randomized trials, especially the reporting of methods used to collect harms data, remains inadequate.

Similar content being viewed by others
Abbreviations
- CONSORT:
-
Consolidated standards of reporting trials
- RCTs:
-
Randomized control trials
References
Bagul NB, Kirkham JJ. The reporting of harms in randomized controlled trials of hypertension using the CONSORT criteria for harm reporting. Clin Exp Hypertens. 2012;34(8):548–54.
de Vries TW, van Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Arch Dis Child. 2010;95(12):1023–6.
Haidich AB, Birtsou C, Dardavessis T, Tirodimos I, Arvanitidou M. The quality of safety reporting in trials is still suboptimal: survey of major general medical journals. J Clin Epidemiol. 2011;64(2):124–35.
Maggi CB, Griebeler IH, Dal Pizzol Tda S. Information on adverse events in randomised clinical trials assessing drug interventions published in four medical journals with high impact factors. Int J Risk Saf Med. 2014;26(1):9–22.
O’Day R, Walton R, Blennerhassett R, Gillies MC, Barthelmes D. Reporting of harms by randomised controlled trials in ophthalmology. Br J Ophthalmol. 2014;98(8):1003–8.
Peron J, Maillet D, Gan HK, Chen EX, You B. Adherence to CONSORT adverse event reporting guidelines in randomized clinical trials evaluating systemic cancer therapy: a systematic review. J Clin Oncol. 2013;31(31):3957–63.
Shukralla AA, Tudur-Smith C, Powell GA, Williamson PR, Marson AG. Reporting of adverse events in randomised controlled trials of antiepileptic drugs using the CONSORT criteria for reporting harms. Epilepsy Res. 2011;97(1–2):20–9.
Sivendran S, Latif A, McBride RB, Stensland KD, Wisnivesky J, Haines L, et al. Adverse event reporting in cancer clinical trial publications. J Clin Oncol. 2014;32(2):83–9.
Smith SM, Chang RD, Pereira A, Shah N, Gilron I, Katz NP, et al. Adherence to CONSORT harms-reporting recommendations in publications of recent analgesic clinical trials: an ACTTION systematic review. Pain. 2012;153(12):2415–21.
Williams MR, McKeown A, Pressman Z, Hunsinger M, Lee K, Coplan P, et al. Adverse event reporting in clinical trials of intravenous and invasive pain treatments: an ACTTION systematic review. J Pain. 2016;17(11):1137–49.
Pitrou I, Boutron I, Ahmad N, Ravaud P. Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med. 2009;169(19):1756–61.
Ioannidis JP, Contopoulos-Ioannidis DG. Reporting of safety data from randomised trials. Lancet. 1998;352(9142):1752–3.
Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285(4):437–43.
Ioannidis JP, Evans SJ, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–8.
Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Stephanie Hum, Nader Shaikh and Su Golder have no conflicts of interest that are directly relevant to the content of this study.
Financial disclosures
The authors have no financial relationships relevant to this article to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hum, S.W., Golder, S. & Shaikh, N. Inadequate harms reporting in randomized control trials of antibiotics for pediatric acute otitis media: a systematic review. Drug Saf 41, 933–938 (2018). https://doi.org/10.1007/s40264-018-0680-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0680-0