Abstract
Background
Gabapentin and pregabalin are widely used as antineuropathic pain drugs. Their use is also associated with the development of adverse drug reactions (ADRs), mainly neuropsychiatric.
Objective
The aim of this work was to study ‘serious’ and/or ‘unexpected’ adverse reactions associated with pregabalin and gabapentin.
Study Design
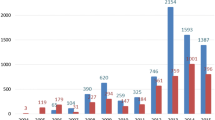
We studied ADRs reported to the French Pharmacovigilance System occurring between 1995 and 2009.
Main Outcome Measure
For each ADR associated with gabapentin or pregabalin, we noted year, patient age and sex, type of adverse reaction, as well as the imputability score. Reporting rate of serious ADRs for gabapentin and pregabalin was estimated with regard to data of use (obtained from the French National Health Insurance Fund) using the defined daily dose. A global and descriptive analysis of the adverse reactions for each drug is presented. Secondly, details of deaths and ADRs with an imputability score of at least ‘probable’ or ‘likely’ were presented.
Results
Overall, 1333 cases were recorded (725 related to gabapentin, 608 related to pregabalin), mainly neuropsychiatric ADRs. Among the 22 deaths recorded, 8 were related to gabapentin in obstetrical situations. Other less well-documented ADRs were identified, such as hepatitis associated with gabapentin and haematological ADRs associated with pregabalin.
Conclusion
This study confirmed the prevalence of neuropsychiatric ADRs associated with gabapentin or pregabalin. A high rate of death occurred with gabapentin in an obstetrical context. New adverse reactions have been noted, such as haematological or hepatic adverse reactions associated with pregabalin and gabapentin, respectively.

Similar content being viewed by others
References
Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105(6):1805–15.
Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin: calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain. 2009;142(1–2):13–6.
Besag FM, Berry D. Interactions between antiepileptic and antipsychotic drugs. Drug Saf. 2006;29(2):95–118.
Buvanendran A, Kroin JS, Della Valle CJ, et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110(1):199–207.
Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Can J Anaesth. 2006;53(5):461–9.
Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain: a systematic review of randomized controlled trials. Pain. 2006;126(1–3):91–101.
Hurley RW, Cohen SP, Williams KA, et al. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31(3):237–47.
Wiffen P, Collins S, McQuay H, et al. Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev 2005; (3): CD001133.
Moore RA, Straube S, Wiffen PJ, et al. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev 2009; (3): CD007076.
Zaccara G, Gangemi P, Perucca P, et al. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011;52(4):826–36.
Khurana DS, Riviello J, Helmers S, et al. Efficacy of gabapentin therapy in children with refractory partial seizures. J Pediatr. 1996;128(6):829–33.
Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109(1–2):26–35.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9.
Montastruc JL, Sommet A, Lacroix I, et al. Pharmacovigilance for evaluating adverse drug reactions: value, organization, and methods. Joint Bone Spine. 2006;73(6):629–32.
Durrieu G, Olivier P, Bagheri H, et al. Overview of adverse reactions to nefopam: an analysis of the French Pharmacovigilance database. Fundam Clin Pharmacol. 2007;21(5):555–8.
Fuzier R, Lapeyre-Mestre M, Samii K, et al. Adverse drug reactions to local anaesthetics: a review of the French pharmacovigilance database. Drug Saf. 2009;32(4):345–56.
Tavassoli N, Lapeyre-Mestre M, Sommet A, et al. Reporting rate of adverse drug reactions to the French pharmacovigilance system with three step 2 analgesic drugs: dextropropoxyphene, tramadol and codeine (in combination with paracetamol). Br J Clin Pharmacol. 2009;68(3):422–6.
Begaud B, Evreux JC, Jouglard J, et al. Unexpected or toxic drug reaction assessment (imputation): actualization of the method used in France. Therapie. 1985;40:111–8. (in French).
Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on addendum to E2C clinical safety data management: periodic safety update reports for marketed drugs; availability. Notice. Fed Regist. 2004;69(24):5551–2.
The WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2011 [online]. Available from URL: http://www.whocc.no/atc_ddd_index/. Accessed 2010 Dec 21.
Bozikas VP, Garyfallos G, Nikolaidis N, et al. Pregabalin induced neutropenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):907–8.
Bates D, Kirby S, Louw A. Thrombocytopenia possibly induced by pregabalin. Can J Hosp Pharm. 2008;61(6):446–7.
Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357(6):580–7.
Richardson CE, Williams DW, Kingham JG. Gabapentin induced cholestasis. BMJ. 2002;325(7365):635.
Bureau C, Poirson H, Peron JM, et al. Gabapentine-induced acute hepatitis. Gastroenterol Clin Biol. 2003;27(12):1169–70.
Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61(3):246–55.
Thiessard F, Roux E, Miremont-Salame G, et al. Trends in spontaneous adverse drug reaction reports to the French pharmacovigilance system (1986–2001). Drug Saf. 2005;28(8):731–40.
Rosa F. Holoprosencephaly and antiepileptic exposures. Teratology. 1995;51(4):230.
Montouris G. Gabapentin exposure in human pregnancy: results from the Gabapentin Pregnancy Registry. Epilepsy Behav. 2003;4(3):310–7.
Molgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA. 2011;305(19):1996–2002.
Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77(2):193–8.
Jentink J, Loane MA, Dolk H, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362(23):2185–93.
Jentink J, Dolk H, Loane MA, et al. Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case-control study. BMJ. 2010;341:c6581.
Harden CL, Meador KJ, Pennell PB, et al. Management issues for women with epilepsy: focus on pregnancy (an evidence-based review). II: teratogenesis and perinatal outcomes. Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1237–46.
Weber JCP. Epidemiology of adverse reactions to non-steroidal antiinflammatory drugs. In: Rainsford KD, Velo GP, editors. Advances in inflammatory research. New York: Raven Press; 1984. p. 1–7.
Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004;24(6):743–9.
Wiffen PJ, McQuay HJ, Edwards JE, et al. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev 2005; (3): CD005452.
Fischer JH, Barr AN, Rogers SL, et al. Lack of serious toxicity following gabapentin overdose. Neurology. 1994;44(5):982–3.
Spiller HA, Bratcher R, Griffith JR. Pregabalin overdose with benign outcome. Clin Toxicol (Phila). 2008;46(9):917.
Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118–22.
Caster O, Edwards IR, Noren GN, et al. Earlier discovery of pregabalin’s dependence potential might have been possible. Eur J Clin Pharmacol. 2011;67(3):319–20.
Grosshans M, Mutschler J, Hermann D, et al. Pregabalin abuse, dependence, and withdrawal: a case report. Am J Psychiatry. 2010;167(7):869.
Schwan S, Sundstrom A, Stjernberg E, et al. A signal for an abuse liability for pregabalin: results from the Swedish spontaneous adverse drug reaction reporting system. Eur J Clin Pharmacol. 2010;66(9):947–53.
Rouve N, Bagheri H, Telmon N, et al. Prescribed drugs and violence: a case/noncase study in the French PharmacoVigilance Database. Eur J Clin Pharmacol. 2011;67(11):1189–98.
Acknowledgements
The authors acknowledge the assistance of all 31 regional centres of the French Pharmacovigilance System. The authors would like to thank Dr Ian Harper, MD (Newcastle, UK), for his very helpful comments regarding the manuscript. No funding was provided for the conduct of the study and/or preparation of the article. There were no relevant conflicts of interest for all authors. All authors discussed the results and commented on the manuscript. R. Fuzier conceived and planned the work, and played an important role in the analysis and interpretation of the data. He wrote the paper. I. Serres played an important role in the acquisition, analysis and interpretation of the data. M. Lapeyre-Mestre and J.L. Montastruc provided substantial contributions to the conception and design, analysis and interpretation of data, revising the manuscript critically for important intellectual content. All authors approved the final submitted version of the manuscript. E. Guitton made substantive suggestions for revision.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Fuzier, R., Serres, I., Guitton, E. et al. Adverse Drug Reactions to Gabapentin and Pregabalin. Drug Saf 36, 55–62 (2013). https://doi.org/10.1007/s40264-012-0006-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-012-0006-6