Abstract
Background
Valproic acid, a histone deacetylase inhibitor, has beneficial effects in the setting of cancer, neurologic diseases, and traumatic injuries. In animal models of traumatic injury, a single dose of valproic acid has been shown to reduce mortality. The purpose of this trial was to determine the maximum tolerated single dose of intravenous valproic acid in healthy humans.
Methods
A double-blinded, placebo-controlled, dose-escalation trial design was used to identify dose-limiting toxicities in healthy subjects who received a single dose of intravenous valproic acid. Patients were monitored for adverse events and data were collected for pharmacokinetic, pharmacodynamic, and safety profiling of valproic acid.
Results
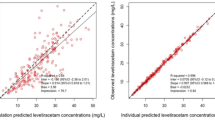
Fifty-nine healthy subjects (mean 30 ± 12 years) were enrolled. Forty-four subjects received valproic acid in doses from 15 to 150 mg/kg. The most common adverse events were hypoacusis (n = 19), chills (n = 18), and headache (n = 16). The maximum tolerated dose was 140 mg/kg. Dose-limiting toxicities included headache and nausea lasting longer than 12 h. No drug-related abnormalities were seen in other safety measures including laboratory tests, hemodynamic parameters, cardiac rhythm monitoring, and cognitive testing. A two-compartment model was predictive of valproic acid concentration–time profiles, with a strong correlation (R 2 = 0.56) observed between the number of reported adverse events and the dose level.
Conclusions
The maximum tolerated dose of intravenous valproic acid in healthy subjects is 140 mg/kg. This is significantly higher than the previously established maximum tolerated dose of 60–75 mg/kg. Next, the safety and tolerability of high-dose valproic acid will be tested in trauma patients in hemorrhagic shock.
ClinicalTrials.gov Identifier: NCT01951560




Similar content being viewed by others
References
Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16(10):695–714.
Gottlicher M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol. 2004;83(Suppl. 1):S91–2.
Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349–52.
Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–40.
West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–9.
Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7(10):854–68.
Toussirot E, Khan KA, Herbein G. Histone deacetylase inhibitors: a new and promising drug class for the treatment of arthritis? Clin Epigenetics. 2010;1(1–2):3–6.
Halaweish I, Nikolian V, Georgoff P, et al. Creating a “prosurvival phenotype” through histone deacetylase inhibition: past, present, and future. Shock. 2015;44(Suppl. 1):6–16.
Boyum A. Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology. 1977;10(2):71–6.
Shackford SR, Mackersie RC, Holbrook TL, et al. The epidemiology of traumatic death: a population-based analysis. Arch Surg. 1993;128(5):571–5.
Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82.
Pons PT, Jerome J, McMullen J, et al. The Hartford consensus on active shooters: implementing the continuum of prehospital trauma response. J Emerg Med. 2015;49(6):878–85.
Alam HB, Shuja F, Butt MU, et al. Surviving blood loss without blood transfusion in a swine poly-trauma model. Surgery. 2009;146(2):325–33.
Fukudome EY, Kochanek AR, Li Y, et al. Pharmacologic resuscitation promotes survival and attenuates hemorrhage-induced activation of extracellular signal-regulated kinase 1/2. J Surg Res. 2010;163(1):118–26.
Li Y, Liu B, Fukudome EY, et al. Surviving lethal septic shock without fluid resuscitation in a rodent model. Surgery. 2010;148(2):246–54.
Dekker SE, Bambakidis T, Sillesen M, et al. Effect of pharmacologic resuscitation on the brain gene expression profiles in a swine model of traumatic brain injury and hemorrhage. J Trauma Acute Care Surg. 2014;77(6):906–12 discussion 12.
Zhao T, Li Y, Liu B, et al. Novel pharmacologic treatment attenuates septic shock and improves long-term survival. Surgery. 2013;154(2):206–13.
Halaweish I, Bambakidis T, Chang Z, et al. Addition of low-dose valproic acid to saline resuscitation provides neuroprotection and improves long-term outcomes in a large animal model of combined traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2015;79(6):911–9.
Jepsen CH, deMoya MA, Perner A, et al. Effect of valproic acid and injury on lesion size and endothelial glycocalyx shedding in a rodent model of isolated traumatic brain injury. J Trauma Acute Care Surg. 2014;77(2):292–7.
Jin G, Duggan M, Imam A, et al. Pharmacologic resuscitation for hemorrhagic shock combined with traumatic brain injury. J Trauma Acute Care Surg. 2012;73(6):1461–70.
Gugler R, von Unruh GE. Clinical pharmacokinetics of valproic acid. Clin Pharmacokinet. 1980;5(1):67–83.
Atmaca A, Al-Batran SE, Maurer A, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer. 2007;97(2):177–82.
Munster P, Marchion D, Bicaku E, et al. Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J Clin Oncol. 2007;25(15):1979–85.
Brown E. Medical dictionary for regulatory activities (MedDRA®). In: Mann RD, Andrews EB, editors. Pharmacovigilance. 2nd ed. Chichester: John Wiley & Sons Ltd; 2006.
Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing. 1972;1(4):233–8.
Folstein MF, Folstein SE, McHugh PR. ”“Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Georgoff P, Halaweish I, Nikolian V, et al. Alterations in the human proteome following administration of valproic acid. J Trauma Acute Care Surg. 2016;81(6):1020–7.
Spiller HA, Krenzelok EP, Klein-Schwartz W, et al. Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol. 2000;38(7):755–60.
Higgins GA, Georgoff P, Nikolian V. Network reconstruction reveals that valproic acid activates neurogenic transcriptional progamsin adult brain following traumatic injury. 2017;. doi:10.1007/s11095-017-2130-6 [Epub ahead of print].
Nikolian V, Dekker SE, Bamdakidis T, et al. Pharmacologic resuscitation decreases blood-brain barrier permeability in a porcine model of traumatic brain injury and hemorrhagic shock. Washington, DC: American College of Surgeons; 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the Department of Defense Office of Naval Research (N000141310071) and the National Institutes of Health Center for Advancing Translational Sciences (UL1TR000433). The Michigan Institute for Clinical Health Research provided guidance, data design, and clinical research space. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or the US Department of Defense.
Conflict of interest
Patrick E. Geogoff, Vahagn C. Nikolian, Tess Bonham, Manjunath P. Pai, Celia Tafatia, Ihab Halaweish, Kathleen To, Kuanwong Watcharotone, Aishwarya Parameswaran, Ruijuan Lao, Duxin Sun, and Hasan B. Alam have no conflicts of interest directly relevant to the contents of this study.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Georgoff, P.E., Nikolian, V.C., Bonham, T. et al. Safety and Tolerability of Intravenous Valproic Acid in Healthy Subjects: A Phase I Dose-Escalation Trial. Clin Pharmacokinet 57, 209–219 (2018). https://doi.org/10.1007/s40262-017-0553-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0553-1