Abstract
Background and Objective
Leuprolide is a gonadotropin-releasing hormone (GnRH) agonist, which inhibits gonadotropin secretion by down-regulating pituitary GnRH receptor when administered continuously at therapeutic doses. The objectives of this analysis were to develop a population model that can describe the pharmacokinetics of the 6-month depot formulation of leuprolide acetate in patients with prostate cancer and to characterize the relationship of leuprolide plasma concentrations and serum testosterone concentrations.
Methods
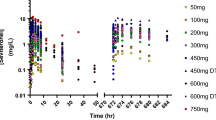
The pharmacokinetic and pharmacodynamic analyses were performed using a non-linear mixed-effect modeling approach. Observations were pooled from studies on healthy male volunteers and prostate cancer patients, who were administered a single 1 mg intravenous dose of immediate-release leuprolide acetate and two intramuscular doses of 45 mg of the depot formulation, respectively. The covariates that were screened for the pharmacokinetic model included body weight, creatinine clearance, liver function markers (total bilirubin, blood urea nitrogen, AST, alanine aminotransferase), age, and body mass index.
Results
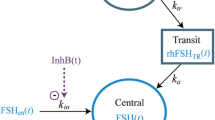
A two-compartment model with parallel first- and zero-order absorption processes and a delayed first-order process well-characterized the multi-phasic absorption profile of leuprolide acetate depot formulation. Typical population values of the absorption rate constant of the immediate and delayed processes were estimated to be 0.357 and 0.017 day−1, respectively, with a mean transit time of 9.5 days. No covariates were significant in this analysis. A semi-mechanistic model, which accounts for down-regulation of the GnRH receptor via an inhibitory maximum effect (E max) model and the stimulatory effect of activated receptors on testosterone levels, adequately described serum testosterone profiles following dosing. The equilibrium dissociation constant of leuprolide and the typical leuprolide plasma concentration required to achieve a castration testosterone level of ≤0.5 ng/mL were 0.3 and 0.03 ng/mL, respectively.
Conclusion
Population pharmacokinetics and pharmacodynamics of the leuprolide depot formulation were characterized using an integrated semi-mechanistic model. The developed model adequately describes the leuprolide–testosterone relationship and can potentially be used to facilitate design of clinical studies for new formulations, to aid in the selection of candidate formulations, and for the optimization of doses and dosing schemes.




Similar content being viewed by others
References
American Cancer Society. What are the key statistics about prostate cancer? 2014. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Accessed 8 Nov 2014.
National Cancer Institute. Prostate cancer treatment (PDQ®). 2014. http://www.cancer.gov/cancertopics/pdq/treatment/prostate/HealthProfessional/page7. Accessed 8 Nov 2014.
Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8:211–23.
Trachtenberg J. Hormonal management of stage D carcinoma of the prostate. In: Carson CC, editor. Problems in urology, vol. 7. Philadelphia: JB Lippincott Co; 1993. p. 215–25.
Perrin MH, Rivier JE, Vale WW. Radioligand assay for gonadotropin-releasing hormone: relative potencies of agonists and antagonists. Endocrinology. 1980;106:1289–93.
US Food and Drug Administration. Lupron Depot label information. 2014. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed 8 Nov 2014.
Conn PM, Roger SC, Seay SG. Biphasic regulation of gonadotropin-releasing hormone receptor by receptor microaggregation and intracellular Ca2+ levels. Med Pharmacol. 1984;25:51–5.
Romero E, Vélez de Mendizabal N, Cendrós J-M, Peraire C, Bascompta E, Obach R, et al. Pharmacokinetic/pharmacodynamic model of the testosterone effects of triptorelin administered in sustained release formulations in patients with prostate cancer. J Pharmacol Exp Ther. 2012;342(3):788–98.
Fattinger KE, Verotta D, Porchet HC, Munafo A, Le Cotonnec JY, Sheiner LB. Modeling a bivariate control system: LH and testosterone response to the GnRH antagonist antide. Am J Physiol. 1996;271(4 Pt 1):E775–87.
Pechstein B, Nagaraja NV, Hermann R, Romeis P, Locher M, Derendorf H. Pharmacokinetic-pharmacodynamic modeling of testosterone and luteinizing hormone suppression by cetrorelix in healthy volunteers. J Clin Pharmacol. 2000;40(3):266–74.
Wong SL, Lau DT-W, Baughman SA, Fotheringham N, Menchaca D, Garnick MB. Pharmacokinetics and pharmacodynamics of a novel depot formulation of abarelix, a gonadotropin-releasing hormone (GnRH) antagonist, in healthy men ages 50 to 75. J Clin Pharmacol. 2004;44(5):495–502.
Tornoe CW, Agers H, Senderovitz T, Nielsen HA, Madsen H, Karlsson MO, et al. Population pharmacokinetic/pharmacodynamic (PK/PD) modeling of the hypothalamic-pituitary-gonadal axis following treatment with GnRH analogues. Br J Clin Pharmacol. 2007;63(6):648–64.
Jadhav PR, Agersø H, Tornøe CW, Gobburu JVS. Semi-mechanistic pharmacodynamic modeling for degarelix, a novel gonadotropin releasing hormone (GnRH) blocker. J Pharmacokinet Pharmacodyn. 2006;33(5):609–34.
Spitz A, Young JM, Larsen L, Mattia-Goldberg C, Donnelly J, Chwalisz K. Efficacy and safety of leuprolide acetate 6-month depot for suppression of testosterone in patients with prostate cancer. Prostate Cancer Prostatic Dis. 2012;15(1):93–9.
Sennello LT, Finley RA, Chu SY, Jagst C, Max D, Rollins DE, et al. Single-dose pharmacokinetics of leuprolide in humans following intravenous and subcutaneous administration. J Pharm Sci. 1986;75(2):158–60.
Mostafa NM, Chwalisz K, Larsen L, Mattia-Goldberg C, Spitz A, Pradhan RS. Evaluation of the pharmacokinetics and pharmacodynamics of two leuprolide acetate 45 mg 6-month depot formulations in patients with prostate cancer. Clin Pharmacol Drug Dev. 2014;3(4):270–5.
Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–13.
Periti P, Mazzei T, Mini E. Clinical pharmacokinetics of depot leuprorelin. Clin Pharmacokinet. 2002;41(7):485–504.
Okada H, Inoue Y, Heya T, Ueno H, Ogawa Y, Toguchi H. Pharmacokinetics of once-a-month injectable microspheres of leuprolide acetate. Pharm Res. 1991;8(6):787–91.
Okada H, Doken Y, Ogawa Y, Toguchi H. Preparation of three-month depot injectable microspheres of leuprorelin acetate using biodegradable polymers. Pharm Res. 1994;11(8):1143–7.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26.
Wilkins JJ, Savic RM, Karlsson MO, Langdon G, McIlleron H, Pillai G, et al. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob Agents Chemother. 2008;52(6):2138–48.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Mosteller R. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
Labrie F. Hormonal therapy of prostate cancer. Prog Brain Res. 2010;182:321–41.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Zlotta A, Debruyne FMJ. Expert opinion on optimal testosterone control in prostate cancer. Eur Urol Suppl. 2005;4:37–41.
Okada H. One- and three-month release injectable microspheres of the LH–RH superagonist leuprorelin acetate. Adv Drug Deliv Rev. 1997;28(1):43–70.
Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 1995;6(4):332–51.
Mazzei T, Mini E, Rizzo M, Periti P. Human pharmacokinetic and pharmacodynamic profiles of leuprorelin acetate depot in prostatic cancer patients. J Int Med Res. 1990;18(Suppl 1):42–56.
Muller FO, Terblanche J, Schall R, van Zyl Smit R, Tucker T, Marais K, et al. Pharmacokinetics of triptorelin after intravenous bolus administration in healthy males and in males with renal or hepatic insufficiency. Br J Clin Pharmacol. 1997;44(4):335–41.
Cockshott ID. Clinical pharmacokinetics of goserelin. Clin Pharmacokinet. 2000;39(1):27–48.
Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13(1):241–5.
Fujii Y, Yonese J, Kawakami S, Yamamoto S, Okubo Y, Fukui I. Equivalent and sufficient effects of leuprolide acetate and goserelin acetate to suppress serum testosterone levels in patients with prostate cancer. BJU Int. 2008;101(9):1096–100.
Lee S-H, Lee H-M, Kim S-W, Lee E-S, Hong S-J, Kim C-S, et al. Is high-dose leuprorelin acetate effective and safe in Asian men with prostate cancer? An open-label, non-comparative, multi-center clinical trial. Yonsei Med J. 2014;55(2):310.
Van der Sluis TM, van Moorselaar RJA, Meuleman EJH, ter Haar RW, Bui HN, Heijboer AC, et al. Relationship between body mass index and serum testosterone concentration in patients receiving luteinizing hormone-releasing hormone agonist therapy for prostate cancer. Urology. 2013;81(5):1005–9.
Gries JM, Munafo A, Porchet HC, Verotta D. Down-regulation models and modeling of testosterone production induced by recombinant human choriogonadotropin. J Pharmacol Exp Ther. 1999;289(1):371–7.
Struthers RS, Xie Q, Sullivan SK, Reinhart GH, Kohout TA, Zhu YF, et al. Pharmacological characterization of a novel nonpeptide antagonist of the human gonadotropin-releasing hormone receptor, NBI-42902. Endocrinology. 2007;148(2):857–67.
Khan MS, O’Brien A. An evaluation of pharmacokinetics and pharmacodynamics of leuprorelin acetate 3M-depot in patients with advanced and metastatic carcinoma of the prostate. Urol Int. 1998;60(1):33–40.
Disclosure statement
The design, study conduct, and financial support for the clinical trials were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication. Both authors are employees of AbbVie and have no additional conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, C.N., Salem, A.H. A Semi-Mechanistic Integrated Pharmacokinetic/Pharmacodynamic Model of the Testosterone Effects of the Gonadotropin-Releasing Hormone Agonist Leuprolide in Prostate Cancer Patients. Clin Pharmacokinet 54, 963–973 (2015). https://doi.org/10.1007/s40262-015-0251-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0251-9