Abstract
Objective
Pregabalin is a gamma aminobutyric acid derivative administered for neuropathic pain. It binds to α2δ subunits of voltage-dependent calcium channels, and inhibits calcium inflow of synapses and the release of excitatory neurotransmitters. This study investigated the efficacy and safety of pregabalin in patients with peripheral neuropathic pain undergoing maintenance hemodialysis.
Methods
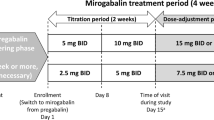
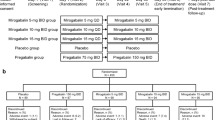
This study was a prospective, open-label, single-arm, multi-center trial. Patients were treated with an initial dose of pregabalin at 25 mg; this was then increased up to a maximum of 150 mg depending on the patient during a 12-week study period. Visual Analog Scale, Eight-Item Short Form Health Survey (SF-8), and laboratory data were collected at baseline and the end of the study.
Results
A total of 45 patients with peripheral neuropathic pain were included, of whom 35 patients were analyzed. The final mean dose of pregabalin was 50.7 mg daily. Mean Visual Analog Scale scores significantly decreased from 52.4 mm at baseline to 34.1 mm at the end of the study (p < 0.0001). Scores for all eight categories of the SF-8 significantly increased compared with baseline (p < 0.05). Both physical and mental component summary scores of the SF-8 also significantly increased (p < 0.05). Ten patients were withdrawn from the study because of drowsiness, dizziness, and invalidity; however, no serious adverse drug reactions were recorded.
Conclusions
If adverse effects are carefully monitored and the administered dosage prudently determined, pregabalin can be an effective treatment for peripheral neuropathic pain in patients undergoing hemodialysis.
Trial Registration: UMIN000023117.




Similar content being viewed by others
References
Institute for Clinical Systems Improvement. Health care guideline: assessment and management of chronic pain. 6th ed., November 2013. http://www.icsi.org/_asset/bw798b/ChronicPain.pdf. Accessed 22 Aug 2016.
Merskey H, Bogduk N, editors. IASP Task Force on taxonomy, classification of chronic pain. 2nd ed. Seattle: IASP Press; 1994. p. 209–14.
Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101:17–24.
Cruccu G, Anand P, Attal N, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–62.
Masakane I, Nakai S, Ogata S, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2013). Ther Apher Dial. 2015;19:540–74.
Abe M, Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015;11:302–13.
Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–51.
Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain: consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13–21.
Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–23.
Tan T, Barry P, Reken S, Baker M. Pharmacological management of neuropathic pain in non-specialist settings: summary of NICE guidance. BMJ. 2010;340:707–9.
Randinitis EJ, Posvar EL, Alvey CW, et al. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol. 2003;43:277–83.
Ware J, Kosinski M, Dewey J, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8 Health Survey. Boston: QualyMetric; 2001.
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83.
Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60:1274–83.
Cappuzzo KA. Treatment of postherpetic neuralgia: focus on pregabalin. Clin Interv Aging. 2009;4:17–23.
Arezzo JC, Rosenstock J, LaMoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. doi:10.1186/1471-2377-8-33.
Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2010;28:109–16.
Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin treatment over 52 weeks in patients with diabetic neuropathic pain extended after a double-blind placebo-controlled trial. J Diabetes Investig. 2011;2:457–563.
Navarro A, Saldaña MT, Pérez C, et al. Patient-reported outcomes in subjects with neuropathic pain receiving pregabalin: evidence from medical practice in primary care settings. Pain Med. 2010;11:719–31.
Freynhagen R, Grond S, Schüpfer G, et al. Efficacy and safety of pregabalin in treatment refractory patients with various neuropathic pain entities in clinical routine. Int J Clin Pract. 2007;61:1989–96.
Agarwal A, Gautam S, Gupta D, et al. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth. 2008;101:700–4.
Zhang SS, Wu Z, Zhang LC, et al. Efficacy and safety of pregabalin for treating painful diabetic peripheral neuropathy: a meta-analysis. Acta Anaesthesiol Scand. 2015;59:147–59.
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14:162–73.
Haanpaa M, Treede RD. Diagnosis and classification of neuropathic pain. Pain Clinical Update. 2010;XVIII, Issue 7.
Kehler H, Jensen TS, Woolf C. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25.
Kavoussi R. Pregabalin: from molecule to medicine. Eur Neuropsychopharmacol. 2006;16(Suppl 2):S128–33.
Ohishi A, Chisaki Y, Hira D, et al. Opioid analgesics increase incidence of somnolence and dizziness as adverse effects of pregabalin: a retrospective study. J Pharm Health Care Sci. 2015;1:30. doi:10.1186/s40780-015-0032-5.
Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45(Suppl 6):13–8.
Yue J, Jiao S, Xiao Y, et al. Comparison of pregabalin with ondansetron in treatment of uraemic pruritus in dialysis patients: a prospective, randomized, double-blind study. Int Urol Nephrol. 2015;47:161–7.
Annemans L, Caekelbergh K, Morlion B, et al. A cost-utility analysis of pregabalin in the management of peripheral neuropathic pain. Acta Clin Belg. 2008;63:170–8.
Vranken JH, Dijkgraaf MG, Kruis MR, et al. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received for the preparation of this study.
Conflict of interest
MA has received honoraria from Kyowa Hakko Kirin, Daiichi Sankyo, and Otsuka Pharmaceutical. The other authors declare that they have no relevant financial interests.
Ethical approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration, and the ethical committees of our hospitals approved the study.
Informed consent
Written informed consent was obtained from all patients.
Rights and permissions
About this article
Cite this article
Otsuki, T., Higuchi, T., Yamazaki, T. et al. Efficacy and Safety of Pregabalin for the Treatment of Neuropathic Pain in Patients Undergoing Hemodialysis. Clin Drug Investig 37, 95–102 (2017). https://doi.org/10.1007/s40261-016-0464-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0464-1