Abstract
Background and Objectives
The combination of direct-acting antiviral agents in patients with chronic hepatitis C virus (HCV) infection has demonstrated clinical benefit; however, evaluation of potential drug–drug interactions is required prior to therapy.
Methods
An open-label study assessed the pharmacokinetics and tolerability of the HCV NS5A replication complex inhibitor daclatasvir and the HCV NS3 protease inhibitor asunaprevir when co-administered in healthy subjects. Daclatasvir 60 mg once daily and asunaprevir 600 mg twice daily were dosed for 7 days alone followed by combination dosing for 14 days at 30 mg once daily and 200 mg twice daily, respectively. Further assessments were provided comparing exposures from the current study with those from studies in HCV-infected patients receiving either the same or higher doses of daclatasvir or asunaprevir administered alone or together.
Results
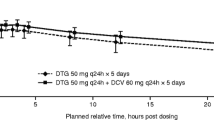
Dose-normalized daclatasvir and asunaprevir morning exposures were comparable with control in healthy subjects, with geometric mean area under the concentration–time curve ratios of 1.202 (90 % CI 1.113–1.298) and 0.868 (90 % CI 0.726–1.038), respectively. In HCV patients daclatasvir and asunaprevir exposures were largely comparable, when administered together or alone.
Conclusions
Additional data support the conclusion that there is no clinically meaningful interaction between daclatasvir and asunaprevir in either healthy subjects or HCV-infected patients, including those also receiving peginterferon-α/ribavirin, and that the combination of daclatasvir 60 mg once daily and asunaprevir 200 mg twice daily is generally well-tolerated.


Similar content being viewed by others
References
Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS-790052, an NS5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology. 2011;54(6):1956–65.
Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465(7294):96–100.
Pelosi LA, Voss S, Liu M, Gao M, Lemm JA. Effect on hepatitis C virus replication of combinations of direct-acting antivirals, including NS5A inhibitor daclatasvir. Antimicrob Agents Chemother. 2012;56(10):5230–9.
Friborg J, Levine S, Chen C, Sheaffer AK, Chaniewski S, Voss S, et al. Combinations of lambda interferon with direct-acting antiviral agents are highly efficient in suppressing hepatitis C virus replication. Antimicrob Agents Chemother. 2013;57(3):1312–22.
Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, et al. Daclatasvir for previously untreated chronic hepatitis C genotype 1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12(9):671–7.
Dore GJ, Lawitz E, Hézode C, Shafran S, Ramji A, Tatum H, et al. Daclatasvir combined with peginterferon alfa-2a and ribavirin for 12 or 16 weeks in patients with HCV genotype 2 or 3 infection: COMMAND GT2/3 study. J Hepatol. 2013;58(Suppl 1):S570–1.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson IM, et al. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naïve patients chronically infected with HCV genotype 1, 2, or 3 [abstract]. Hepatology 2012;56(Suppl 4):abstract LB-02.
Everson GT, Sims KD, Rodriguez-Torres M, Hézode C, Lawitz E, Bourlière M, et al. Interim analysis of an interferon (IFN)- and ribavirin (RBV)-free regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 in treatment-naive, hepatitis C virus genotype 1-infected patients [abstract]. J Hepatol. 2013;58(Suppl 1):S573.
Lok A, Gardiner D, Hézode C, Lawitz E, Bourlière M, Everson G, et al. Randomized trial of daclatasvir and asunaprevir with or without peginterferon/ribavirin for hepatitis C virus genotype 1 null responders. J Hepatol. 2014;60(3):490–9.
McPhee F, Sheaffer AK, Friborg J, Hernandez D, Falk P, Zhai G, et al. Preclinical profile and characterization of the hepatitis C virus NS3 protease inhibitor asunaprevir (BMS-650032). Antimicrob Agents Chemother. 2012;56(10):5387–96.
Suzuki Y, Ikeda K, Suzuki F, Toyota J, Karino Y, Chayama K, et al. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2013;58(4):655–62.
Eley T, Pasquinelli C, Wendelburg P, Villegas C, He B, Liao S, et al. Safety and pharmacokinetics of BMS-650032 following multiple ascending doses for 14 days in healthy subjects [abstract]. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 12–15 Sep 2010; Boston.
Lok AS, Gardiner D, Hezode C, Lawitz E, Bourliere M, Everson G, et al. Sustained virologic response in chronic HCV genotype (GT) 1-infected null responders with combination of daclatasvir (DCV; NS5A inhibitor) and asunaprevir (ASV; NS3 inhibitor) with or without peginterferon alfa-2a/ribavirin (PEG/RBV) [abstract no. 79]. Hepatology. 2012;56(Suppl 4):230A.
Charlton M. Telaprevir, boceprevir, cytochrome P450 and immunosuppressive agents–a potentially lethal cocktail. Hepatology. 2011;54(1):3–5.
Eley T, Gardiner D, Persson A, He B, You X, Shah V, et al. Evaluation of drug interaction potential of the HCV protease inhibitor asunaprevir (ASV; BMS-650032) at 200 mg twice daily (BID) in metabolic cocktail and P-glycoprotein (P-gp) probe studies in healthy volunteers [abstract]. Hepatology 2011;54(Suppl 1):381.
Bifano M, Sevinsky H, Stonier M, Jiang H, Bertz RJ. Daclatasvir, an HCV NS5A replication complex inhibitor, has minimal effect on pharmacokinetics of midazolam, a sensitive probe for cytochrome P450 3A4 [abstract no. O_15]. 8th International Workshop on Clinical Pharmacology of Hepatitis Therapy; 26–27 Jun 2013; Cambridge (MA).
BMS-790052 and BMS-650032: One-month Oral Combination Toxicity Study in Monkeys (DS08143). Bristol-Myers Squibb Research and Development, 2009. BMS Document Control No. 930035546.
Chan P, Tafoya E, Bifano M, Bertz R, Yin P, Hughes E, et al. Exposure-response analysis of daclatasvir in patients with genotype 1 chronic HCV infection: dose selection for phase 3 clinical trials [abstract no. PK-11]. Rev Antivir Ther Infect Dis. 2012;6:14.
Chan P, Tafoya E, Eley T, He B, Mendez P, Gardiner D, et al. Exposure-response analyses of asunaprevir in combination with daclatasvir +/− peginterferon/ribavirin among patients with genotype 1 chronic HCV infection: dose selection for phase 3 clinical trials. J Hepatol. 2013;58(Suppl 1):S328–9.
Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366(3):216–24.
Eley T, He B, Huang S, Li W, Pasquinelli C, Rodrigues AD, et al. Pharmacokinetics of the NS3 protease inhibitor, asunaprevir (ASV, BMS-650032), in phase I studies in subjects with or without chronic hepatitis C. Clin Pharm Drug Dev. 2013;2(4):316–27.
Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. BMS-650032, an NS3 inhibitor, in combination with peginterferon alfa-2a and ribavirin in treatment-naive subjects with genotype 1 chronic hepatitis C infection [abstract]. J Hepatol. 2011;54(Suppl 1):S472.
Vertex Pharmaceuticals Inc. INCIVEK™ (telaprevir) prescribing information. 2013. http://pi.vrtx.com/files/uspi_telaprevir.pdf. Accessed 17 July 2014
Merck & Co., Inc. VICTRELIS™ (boceprevir) prescribing information. 2013. http://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf. Accessed 17 July 2014
Eley T, Chan P, Sverdlov O, He B, Bedford B, Kandoussi H, et al. Improved bioavailability and mitigated food effect for asunaprevir utilizing a lipid-based formulation: similar exposure with 100 mg twice daily softgel capsule relative to 200 mg twice daily of phase 2 tablet [abstract/poster no. A-1247]. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 9–12 Sep 2012; San Francisco (CA).
Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. Randomized study of asunaprevir plus peginterferon alfa and ribavirin for previously untreated genotype 1 chronic hepatitis C. Antivir Ther. 2013;18(7):885–93.
Benet LZ. Predicting drug disposition via application of a biopharmaceutics drug disposition classification system. Basic Clin Pharmacol Toxicol. 2010;106(3):162–7.
Eley T, Han YH, Huang SP, He B, Li W, Bedford B, et al. In vivo and in vitro assessment of asunaprevir (BMS-650032) as an inhibitor and substrate of organic anion polypeptide (OATP) transporters in healthy volunteers. International Workshop on Clinical Pharmacology of Hepatitis Therapy; 27–28 Jun 2012; Cambridge (MA).
Bifano M, Hwang C, Hartstra J, Tiessen RG. Assessment of HIV antiretroviral drug interactions with the HCV NS5A replication complex inhibitor daclatasvir demonstrates a PK profile which supports coadministration with tenofovir, efavirenz and atazanavir/r [poster no. 618]. 19th Conference on Retroviruses and Opportunistic Infections (CROI 2012); 5–8 Mar 2012; Seattle (WA).
Bifano M, Sevinsky H, Persson A, Hwang C, Kandoussi H, Jiang H, et al. BMS-790052 has no effect on the pharmacokinetics of a combined oral contraceptive containing ethinyl estradiol and norgestimate in healthy female subjects [poster no. 1340]. The Liver Meeting 2011: The 62nd Annual Meeting of the American Association for the Study of Liver Diseases; 4–8 Nov 2011; San Francisco (CA).
Fontana RJ, Hughes EA, Appelman H, Hindes R, Dimitrova D, Bifano M. Case report of successful peginterferon, ribavirin, and daclatasvir therapy for recurrent cholestatic hepatitis C after liver retransplantation. Liver Transpl. 2012;18(9):1053–9.
Fontana RJ, Bifano M, Hindes R, Symonds WT, Dimitrova D. First ever successful use of daclatasvir and GS-7977, an interferon-free oral regimen, in a liver transplant recipient with severe recurrent hepatitis C [abstract]. Hepatology. 2012;56(Suppl 4):524A–5A.
Eley T, You X, Huang S, Symonds W, Li W, Sherman D, et al. Evaluation of drug interaction potential between daclatasvir and sofosbuvir. Rev Antivir Ther Infect Dis. 2013;6:16.
Bronowicki J, Pol S, Thuluvath P, Larrey D, Martorell CT, Rustgi VK, et al. Asunaprevir (ASV; BMS-650032), an NS3 protease inhibitor, in combination with peginterferon and ribavirin in treatment-naive patients with genotype 1 chronic hepatitis C infection [abstract no. 1096]. J Hepatol. 2012;56(Suppl 2):S431–2.
Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez-Torres M, Shafran S, et al. Daclatasvir, an NS5A replication complex inhibitor, combined with peginterferon alfa-2a and ribavirin in treatment-naive HCV-genotype 1 or 4 subjects: phase 2b COMMAND-1 SVR12 results [abstract]. Hepatology. 2012;56(Suppl 4):553A–4A.
Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742–8.
Lok A, Gardiner D, Hézode C, Lawitz E, Bourlière M, Everson G, et al. Confirmation that quadruple therapy with daclatasvir (NS5A inhibitor), asunaprevir (NS3 inhibitor) and peginterferon/ribavirin results in high rate of SVR4 in HCV genotype 1 null responders [abstract no. 1415]. J Hepatol 2012;56(Suppl 2):S557.
Pasquinelli C, McPhee F, Eley T, Villegas C, Sandy K, Wendelburg P, et al. Single and multiple ascending dose studies of the NS3 protease inhibitor asunaprevir in subjects with or without chronic hepatitis C. Antimicrob Agents Chemother. 2012;56:1838–44.
Acknowledgments
The authors thank Anna Persson Berglind, formerly of Bristol-Myers Squibb, and John Coumbis at Bristol-Myers Squibb, the research staff at participating sites, and the subjects and their families for their assistance with conducting these studies. Editorial assistance was provided by Andrew Street and Andrew Stead of Articulate Science Ltd. and was funded by Bristol-Myers Squibb.
Conflict of Interest Disclosures
All authors are employees and stockholders of Bristol-Myers Squibb.
Author Contributions
T. Eley and M. Bifano contributed to the design of the study, analyses of the data, and the writing of this manuscript. Further contributions to the study design and data analyses were provided by S.-P. Huang, K. Zhu, D. Gardiner, and D. Grasela; R. Bertz, H. Sevinsky, B. He, and H. Kandoussi contributed to data analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eley, T., Sevinsky, H., Huang, SP. et al. The Pharmacokinetics of Daclatasvir and Asunaprevir Administered in Combination in Studies in Healthy Subjects and Patients Infected with Hepatitis C Virus. Clin Drug Investig 34, 661–671 (2014). https://doi.org/10.1007/s40261-014-0219-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0219-9