Abstract
Background
Healthcare cost savings are closely linked to prescribers’ confidence in and acceptance of the prescription of biosimilar drugs.
Objectives
The aim of this study was to assess the knowledge, experience and opinions of hospital-based and office-based French rheumatologists with regard to biosimilar medicines and to identify the barriers to and possible options to promote their prescription.
Methods
A web-based, self-administered survey was conducted among French rheumatologists from June 8 to August 2, 2015.
Results
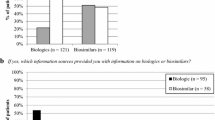
A total of 116 rheumatologists responded to the survey. Many reported having little knowledge and a lack of available information about biosimilar drugs, especially office-based rheumatologists. 98.3% of the respondents had at least one question about biosimilars, and seven in ten raised issues regarding substitution, iatrogenic effects or cost savings that might be achievable. Only eight rheumatologists had already prescribed a biosimilar drug. The most common barriers reported were indication extrapolation and a lack of data about tolerability. Nine out of ten physicians thought that starting a treatment with a biosimilar drug in biologic treatment-naïve patients was possible. The rheumatologists’ opinions were rather favorable towards the implementation of biosimilars, but a majority expressed a negative opinion about substitution by the pharmacist.
Conclusions
Our survey gave a better appreciation of the concerns associated with biosimilar prescriptions. Targeted communication initiatives, deeper experience and availability of new clinical data may help to address the outstanding questions and should overcome the misunderstandings surrounding biosimilar drugs among rheumatologists.





Similar content being viewed by others
References
Braun J, Kudrin A. Progress in biosimilar monoclonal antibody development: the infliximab biosimilar CT-P13 in the treatment of rheumatic diseases. Immunotherapy. 2015;7:73–87. doi:10.2217/imt.14.109.
Weise M, Kurki P, Wolff-Holz E, Bielsky MC, Schneider CK. Biosimilars: the science of extrapolation. Blood. 2014;124:3191–6. doi:10.1182/blood-2014-06-583617.
Weise M, Bielsky M-C, De Smet K, Ehmann F, Ekman N, Giezen TJ, et al. Biosimilars: what clinicians should know. Blood. 2012;120:5111–7. doi:10.1182/blood-2012-04-425744.
Schneider CK. Biosimilars in rheumatology: the wind of change. Ann Rheum Dis. 2013;72:315–8.
Yoo DH. The rise of biosimilars: potential benefits and drawbacks in rheumatoid arthritis. Expert Rev Clin Immunol. 2014;10:981–3. doi:10.1586/1744666X.2014.932690.
Mysler E, Pineda C, Horiuchi T, Singh E, Mahgoub E, Coindreau J, et al. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int. 2016. doi:10.1007/s00296-016-3444-0.
European Medicines Agency (EMA). Guideline on similar biological medicinal products. CHMP/437/04. 23 October 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed 22 Mar 2016.
European Medicines Agency (EMA). Guideline on similar biological medicinal products containing monoclonal antibodies - non-clinical and clinical issues. EMA/CHMP/BMWP/403543/2010. 30 May 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf. Accessed 29 Oct 2015.
Bocquet F. Les Médicaments Biosimilaires: enjeux économiques et politiques. Paris: Éditions de santé; 2015.
Isaacs JD, Cutolo M, Keystone EC, Park W, Braun J. Biosimilars in immune-mediated inflammatory diseases: initial lessons from the first approved biosimilar anti-tumour necrosis factor monoclonal antibody. J Intern Med. 2016;279(1):41–59. doi:10.1111/joim.12432.
Gulácsi L, Brodszky V, Baji P, Kim H, Kim SY, Cho YY, et al. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol. 2015;11(Suppl 1):43–52. doi:10.1586/1744666X.2015.1090313.
FirstWord Pharma. FirstWord Lists—the best selling drugs in 2014. http://www.firstwordpharma.com/node/1263906. Accessed 3 Mar 2016.
Henry D, Taylor C. Pharmacoeconomics of cancer therapies: considerations with the introduction of biosimilars. Semin Oncol. 2014;41(Suppl 3):S13–20. doi:10.1053/j.seminoncol.2014.03.009.
Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM). Les médicaments biosimilaires—État des lieux, 2013. http://www.ansm.sante.fr/var/ansm_site/storage/original/application/6187b427efca64d2a15e496ff691158e.pdf. Accessed 22 Mar 2016.
Kurki P, Ekman N. Biosimilar regulation in the EU. Expert Rev Clin Pharmacol. 2015;8:649–59. doi:10.1586/17512433.2015.1071188.
Tanabe K, Sugimoto N, Fujimoto Y. A web-based survey to investigate the extent of awareness and understanding for biosimilar among Japanese physicians and pharmacists. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2015;18:A658. doi:10.1016/j.jval.2015.09.2381.
EuropaBio. ASBM survey of European prescribers understanding and knowledge of biosimilar medicines. http://www.europabio.org/asbm-survey-european-prescribers-understanding-and-knowledge-biosimilar-medicines. Accessed 3 Mar 2016.
Danese S, Fiorino G, Michetti P. Viewpoint: knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn’s and Colitis Organization. J Crohns Colitis. 2014;8:1548–50. doi:10.1016/j.crohns.2014.06.007.
Alliance for Safe Biologic Medicines (ASBM). Latin American physicians support distinguishable biosimilar naming, survey finds. http://safebiologics.org/resources/2015/06/latin-american-physicians-support-distinguishable-biosimilar-naming-survey-finds/. Accessed 22 Mar 2016.
Institut National de la Statistique et des Etudes Economiques (Insee). Médecins suivant le statut et la spécialité en 2015. http://www.insee.fr/fr/themes/tableau.asp?reg_id=0&ref_id=NATTEF06102. Accessed 4 Mar 2016.
Syndicat National des Médecins Rhumatologues, Société Française de Rhumatologie, Collège Français des Médecins Rhumatologues. Livre Blanc de la Rhumatologie Française 2015. http://sfr.larhumatologie.fr/rc/rhumatologie/htm/Article/2015/sfr-20151001-095142-363/src/htm_fullText/fr/Livre-Blanc-Rhumatologie-2015.pdf. Accessed 17 May 2016.
European Medicines Agency (EMA). Assessment report Inflectra. EMA/CHMP/589422/2013. 27 June 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002778/WC500151490.pdf. Accessed 17 Oct 2016.
European Medicines Agency (EMA). Assessment report Remsima. EMA/CHMP/589317/2013. 27 June 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf. Accessed 17 Oct 2016.
Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–12. doi:10.1136/annrheumdis-2012-203091.
Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–20. doi:10.1136/annrheumdis-2012-203090.
Annese V, Avendaño-Solá C, Breedveld F, Ekman N, Giezen TJ, Gomollón F, et al. Roundtable on biosimilars with European regulators and medical societies, Brussels, Belgium, 12 January 2016. Generics Biosimilars Initiat J (GaBI Journal). 2016;5(2):74–83. doi:10.5639/gabij.2016.0502.019.
ClinicalTrials.gov. “Efficacy and Safety of Infliximab-biosimilar (Inflectra) Compared to Infliximab-innovator (Remicade) in Patients With Inflammatory Bowel Disease in Remission: the SIMILAR Trial”. https://clinicaltrials.gov/ct2/show/NCT02452151. Accessed 4 Mar 2016.
Jung YS, Park DI, Kim YH, Lee JH, Seo PJ, Cheon JH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015;30:1705–12. doi:10.1111/jgh.12997.
Park SH, Kim Y-H, Lee JH, Kwon HJ, Lee S-H, Park DI, et al. Post-marketing study of biosimilar infliximab (CT-P13) to evaluate its safety and efficacy in Korea. Expert Rev Gastroenterol Hepatol. 2015;9(Suppl 1):35–44. doi:10.1586/17474124.2015.1091309.
British Society of Gastroenterology (BSG). Clinical trials updates—PANTS study. http://www.bsg.org.uk/research/clinical-trials-updates/index.html. Accessed 4 Mar 2016.
Nikiphorou E, Kautiainen H, Hannonen P, Asikainen J, Kokko A, Rannio T, et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15:1677–83. doi:10.1517/14712598.2015.1103733.
Sieczkowska J, Jarzębicka D, Banaszkiewicz A, Plocek A, Gawronska A, Toporowska-Kowalska E, et al. Switching between infliximab originator and biosimilar in paediatric patients with inflammatory bowel disease. Preliminary observations. J Crohns Colitis. 2016;10:127–32. doi:10.1093/ecco-jcc/jjv233.
ClinicalTrials.gov. The NOR-SWITCH Study. https://clinicaltrials.gov/ct2/show/NCT02148640. Accessed 4 Mar 2016.
Nederlands Trial Register. Trial info—the effect of switching treatment from innovator infliximab to infliximab biosimilar on efficacy, safety and immunogenicity in patients with rheumatoid arthritis, spondyloarthritis or psoriatic arthritis in daily clinical care—BIO-SWITCH study. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5279. Accessed 4 Mar 2016.
Faccin F, Tebbey P, Alexander E, Wang X, Cui L, Albuquerque T. The design of clinical trials to support the switching and alternation of biosimilars. Expert Opin Biol Ther. 2016;27:1–9. doi:10.1080/14712598.2017.1238454.
Reinisch W, Smolen J. Biosimilar safety factors in clinical practice. Semin Arthritis Rheum. 2015;44:S9–15. doi:10.1016/j.semarthrit.2015.04.005.
Haustein R, de Millas C, Höer A, Häussler H. Saving money in the European healthcare systems with biosimilars. Generics Biosimilars Initiat J. 2012;1:120–6.
Brodszky V, Baji P, Balogh O, Péntek M. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ. 2014;15:65–71. doi:10.1007/s10198-014-0595-3.
Jha A, Upton A, Dunlop WCN, Akehurst R. The budget impact of biosimilar infliximab (Remsima(®)) for the treatment of autoimmune diseases in five European countries. Adv Ther. 2015;32:742–56. doi:10.1007/s12325-015-0233-1.
McCarthy G, Ebel Bitoun C, Guy H. Introduction of an infliximab biosimilar (CT-P13): a five-year budget impact analysis for the treatment of rheumatoid arthritis in Ireland. Value Health. 2013;16:A558. doi:10.1016/j.jval.2013.08.1465.
Lucioni C, Mazzi S, Caporali R. Budget impact analysis of infliximab biosimilar: the Italian scenery. Glob Reg Health Technol Assess. 2015;2:78–88.
Kim J, Hong J, Kudrin A. 5 year budget impact analysis of biosimilar infliximab for the treatment of rheumatoid arthritis in UK, Italy, France and Germany. Arthritis Rheumatol. 2014;11:S512 (abstract 1166).
Beck M, Michel B, Rybarczyk-Vigouret M-C, Sordet C, Sibilia J, Velten M. Biosimilar infliximab for the management of rheumatoid arthritis in France: what are the expected savings? Eur J Hosp Pharm. 2016:ejhpharm–2016–000904. doi:10.1136/ejhpharm-2016-000904.
Acknowledgements
The authors would like to thank all rheumatologists who participated in the pilot study for giving their impressions and advice—Marc Ardizzone, Hélène Bauer, Emmanuel Chatelus, Jacques-Eric Gottenberg, Paul Moreau, Nawal Rahal, Laëtitia Sparsa—and everyone who provided assistance in the survey’s dissemination: the “National Union of Rheumatologists” (Syndicat National des Médecins Rhumatologues) and the “Inflammatory Joint Disease Working Group of the French Society for Rheumatology” (Club “Rhumatismes et Inflammations”). The authors would like to thank Valérie Leray for proofreading and linguistic review of the manuscript. Last but not least, the authors would like to gratefully acknowledge all participants in the survey for completing the questionnaire.
Author contributions
MB conceived and designed the survey. BM, MCRV, DL, CS and MV were involved in validating the survey questionnaire. MB, CS and JS were involved in the survey’s dissemination. MB collected and analyzed the data. All authors checked the accuracy of the results. All authors were involved in drafting the article and revising it critically for important intellectual content. MB acts as guarantor for the content of the paper. All authors read and approved the final manuscript submitted for publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
JS has received grants (<€10,000) from Roche, Pfizer, Abbvie, UCB and consulting fees or honorarium (<€1500) from Roche, Chugai, Bristol Myers Squibb, Abbott, UCB, GSK, LFB, Actelion, Pfizer, Merck Sharp, Novartis, Amgen, Hospira and Abbvie. MB, BM, MCRV, DL, CS and MV declare that they have no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Additional information
J. Sibilia and M. Velten contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beck, M., Michel, B., Rybarczyk-Vigouret, MC. et al. Rheumatologists’ Perceptions of Biosimilar Medicines Prescription: Findings from a French Web-Based Survey. BioDrugs 30, 585–592 (2016). https://doi.org/10.1007/s40259-016-0202-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-016-0202-5