Abstract
Background
Erlotinib has been reported as being associated with a high incidence of skin toxicities such as acneiform rash, paronychia, and xerosis.
Objective
The aim of this study was to evaluate the efficacy of prophylactic minocycline treatment for the skin toxicities induced by erlotinib as compared with deferred minocycline treatment in patients with pancreatic cancer treated with erlotinib plus gemcitabine.
Methods
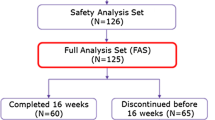
A total of 96 patients were studied retrospectively, of whom 44 received prophylactic minocycline between August 2012 and June 2013 and 52 received deferred minocycline treatment between August 2011 and July 2012 at the National Cancer Center Hospital East, Kashiwa, Japan. In the prophylactic minocycline group, 200 mg/day oral minocycline was prophylactically administered during the treatment period.
Results
The incidence rate of acneiform rash and xerosis of any grade during the first 6 weeks of treatment was significantly reduced in the prophylactic minocycline group compared with the deferred minocycline treatment group (47.7 vs. 80.8 %, p < 0.001; 2.3 vs. 19.2 %, p = 0.01). Multivariate analysis identified prophylactic minocycline as a significant independent factor associated with the incidence of acneiform rash and xerosis of any severity (odds ratio [OR] 0.16, 95 % confidence interval [CI] 0.06–0.46, p < 0.001; OR 0.11, 95 % CI 0.01–0.90, p = 0.04).
Conclusion
Prophylactic minocycline appears to be useful for the management of erlotinib-related acneiform rash and xerosis during chemotherapy in patients with advanced pancreatic cancer.



Similar content being viewed by others
References
Moore MJ, Goldstein D, Hamm J, Figer A, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the national cancer institute of canada clinical trial group. J Clin Oncol. 2007;25:1960–6.
Okusaka T, Furuse J, Funakoshi A, et al. Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci. 2011;102:425–31.
Nakagawa K, Kudoh S, Ohe Y, et al. Postmarketing surveillance study of erlotinib in Japanese patients with non-small-cell lung cancer (NSCLC): an interim analysis of 3488 patients (POLARSTAR). J Thorac Oncol. 2012;7:1296–303.
Galimont-Collen AF, Vos LE, Lavrijsen AP, et al. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer. 2007;43:845–51.
Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390–6.
Lacouture ME, Michell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:1351–7.
Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–21.
Wheatley-Price P, Ding K, Seymour L, et al. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group study BR.21. J Clin Oncol. 2008;26:2350–7.
Jatoi A, Green EM, Rowland KM, et al. Clinical predictors of severe cetuximab-induced rash: observation from 933 patients enrolled in North Central Cancer Treatment Group study N0147. Oncology. 2009;77:120–3.
Perez-Soler R, Chachoua A, Hammond LA, et al. Determinations of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–47.
Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:3238–47.
Nanney LB, Stoscheck CM, King LE Jr, et al. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol. 1990;94:742–8.
Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Targ Oncol. 2009;4:107–19.
Pastore S, Mascia F, Mariotti F, et al. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005;174:5047–56.
Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–12.
Pruzanski W, Greenwald RA, Street IP, et al. Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline. Biochem Pharmacol. 1992;44:1165–70.
Amin AR, Attur MG, Thakker GD, et al. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci. 1996;93:14014–9.
Dreno B, Bettoli V, Ochsendorf F, et al. European recommendations on the use of oral antibiotics for acne. Eur J Dermatol. 2004;14:391–9.
Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–30.
Shu KY, Kindler HL, Medenica M, et al. Doxycycline for the treatment of paronychia induced by the epidermal growth factor receptor inhibitor cetuximab. Br J Dermatol. 2006;154:191–2.
Stintzing S, Kapaun C, Laubender RP, et al. Prognostic value of cetuximab-related skin toxicity in metastatic colorectal cancer patients and its correlation with parameters of the epidermal growth factor receptor signal transduction pathway: results from a randomized trial of the GERMAN AIO CRC Study Group. Int J Cancer. 2013;132:236–45.
Migliaccio A, Castoria G, Di Domenico M, et al. Steroid-induced androgen receptor-oestradiol receptor-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–17.
Migliaccio A, Castoria G, Di Domenico M, et al. Crosstalk between EGFR and extranuclear steroid receptors. Ann NY Acad Sci. 2006;1089:194–200.
Acknowledgments
This manuscript was prepared without the help of any funding source. A. Shinohara, M. Ikeda, H. Okuyama, M. Kobayashi, H. Funazaki, S. Mitsunaga, S. Shimizu, I. Ohno, H. Takahashi, Y. Ichida, K. Takahashi, T. Okusaka and S. Saitoh have no conflicts of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinohara, A., Ikeda, M., Okuyama, H. et al. Efficacy of Prophylactic Minocycline Treatment for Skin Toxicities Induced by Erlotinib Plus Gemcitabine in Patients with Advanced Pancreatic Cancer: A Retrospective Study. Am J Clin Dermatol 16, 221–229 (2015). https://doi.org/10.1007/s40257-015-0116-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-015-0116-x