Abstract
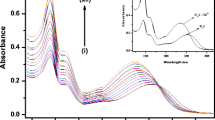
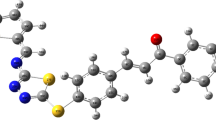
In this paper, a novel compound 3-(2-quinolyl)-5-ferrocenyl-isoxazole(5) with high selectivity toward Cu2+ over other heavy and transition-metal(HTM) ions was designed and synthesized in good yields. The compound not only could be used as an electrochemical probe for Cu2+ with an anodic peak shift of Fe(II)/Fe(III) redox couple, but also could be a colorimetric and fluorescent probe due to the detectable change in color by naked eyes and a significant fluorescence quenching of monomeric anthracene moiety. This highly selective sensing of Cu2+ may be attributed to the unprecedented intermolecular electron-transfer reorganization after the oxidation of the first single electron of compound 5, as indicated by electrospray ionization mass spectrometry(ESI-MS) and density functional theory(DFT) calculation results. To the best of our knowledge, this class of compounds have rarely been reported in the field of molecular sensing. It may have a potential significance for the application of the ferrocenyl-isoxazole derivative in molecular recognition.
Similar content being viewed by others
References
de Silva A. P., Nimal G. H. Q., Gunnlaugsson T., Huxley A. J. M., McCoy C. P., Rademacher J. T., Rice T. E., Chem. Rev., 1997, 97, 1515
Beer P. D., Gale P. A., Angew. Chem. Int. Ed., 2001, 40, 486
Beer P. D., Hayes E. J., Coord. Chem. Rev., 2003, 240, 167
Yoon J., Kim S. K., Jiten S. N., Kim K. S., Chem. Soc. Rev., 2006, 35, 355
Kim J. S., Quang D. T., Chem. Rev., 2007, 107, 3780
Zapata F., Caballero A., Espinosa A., Tárraga A., Molina P., Org. Lett., 2008, 10, 41
Zapata F., Caballero A., Espinosa A., Tárraga A., Molina P., Dyad. Inorg. Chem., 2009, 48, 11566
Zapata F., Caballero A., Espinosa A., Tárraga A., Molina P., J. Org. Chem., 2009, 74, 4787
Alfonso M., Espinosa A., Tárraga A., Molina P., Dyad. Org. Lett., 2011, 13, 2078
Alfonso M., Tárraga A., Molina P., Inorg. Chem., 2013, 52, 7487
Thakur A., Sardar S., Ghosh S., Inorg. Chem., 2011, 50, 7066
Thakur A., Ghosh S., Organometallics, 2012, 31, 819
Thakur A., Mandal D., Ghosh S., Anal. Chem., 2013, 85, 1665
Multhaup G., Schlicksupp A., Hesse L., Beher D., Ruppert T., Masters C. L., Beyreuther K., Science, 1996, 271, 1406
Løvstad R. A., BioMetals, 2004, 17, 111
Amendola V., Boiocchi M., Brega V., Fabbrizzi L., Mosca L., Inorg. Chem., 2010, 49, 997
Ballesteros E., Moreno D., Torroba T., Org. Lett., 2009, 11, 1269
Brunner J., Kraemer R., J. Am. Chem. Soc., 2004, 126, 13626
Zeng L., Miller E. W., Pralle A., Isacoff E. Y., Chang C. J., J. Am. Chem. Soc., 2006, 128, 10
Chin J., Kim K. M., J. Am. Chem. Soc., 2008, 130, 12606
Xie Y. S., Ding Y. B., Li X., Wang C., Hill J. P., Ariga K., Zhang W. B., Zhu W. H., Chem. Commun., 2012, 48, 11513
Guan J., Zhang P., Wei T., Lin Q., Yao H., Zhang Y., Chem. Res. Chinese Universities, 2015, 31(3), 347
Frisch M. J., Trucks G. W., Schlegel H. B., Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2009
Marder S. R., Perry J. W., Tiemann B. G., Organometallics, 1991, 10, 1896
Coe B. J., Jones C. J., McCleverty J. A., J. Organomet. Chem., 1994, 464, 225
Muller T. J. J., Netz A., Ansorge M., Organometallics, 1999, 18, 5066
Demas J. N., Crosby G. A., J. Phys. Chem., 1971, 75, 991
Chen B., Ding Y. B., Li X., Zhu W. H., Hill J. P., Arigac K., Xie Y. S., Chem. Commun., 2013, 49, 10136
Ding Y. B., Li X., Li T., Zhu W. H., Xie Y. S., J. Org. Chem., 2013, 78, 5328
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(Nos.21172001, 21372008), the Natural Science Foundation of the Education Administration of Anhui Province, China(No.KJ2016A267), the Special and Excellent Research Fund of Anhui Normal University, China and the Doctoral Scientific Research Foundation of Anhui Normal University, China(No.2016XJ J110).
Electronic supplementary material
40242_2017_6239_MOESM1_ESM.pdf
Ferrocenyl-isoxazole derivative: a novel electrochemical, colorimetric and fluorescent multiple signal probe for highly selective recognition of Cu2+ ions
Rights and permissions
About this article
Cite this article
Zhang, Z., He, X., Shang, Y. et al. Ferrocenyl-isoxazole derivative: a novel electrochemical, colorimetric and fluorescent multiple signal probe for highly selective recognition of Cu2+ ions. Chem. Res. Chin. Univ. 33, 31–35 (2017). https://doi.org/10.1007/s40242-017-6239-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-017-6239-2